
New cystic fibrosis drug now available through NHS England
On October 24, 2019, cystic fibrosis (CF) patients rejoiced at the news that three years of talks between the UK’s National Institute for Health and Care Excellence (NICE) and Vertex Pharmaceuticals had succeeded in a compromise which could see about 5,000 patients gaining access to a potentially life-saving drug called Orkambi within the next month. Orkambi is perceived to be a ‘wonder drug’ because it helps these patients to breathe easier and better, and can be started as young as two years.
Cystric fibrosis 3d medical vector illustration. Image Credit: medicalstocks / Shutterstock
Negotiating for a fair deal
The previous stalemate, in place since 2016, was because NICE thought the drug too high-priced to offer through the NHS. NHS England is the national healthcare system for the UK. Vertex rejected the counter-offer by NICE as being too low to fund more research, and asked the national body to act “in the best interest” of patients with CF. Patients were devastated at the delay, since CF drastically reduces life expectancy, making such decisions urgent issues from their perspective.
This was reflected in the communication sent by the UK’s health ministers, Steve Brine and Lord O’Shaughnessy, to Vertex senior vice president Simon Bedson. It says, in part, “Time really is of the essence. We ask you as a matter of the utmost urgency to proceed with negotiations [to secure] access to your medicines currently licensed in the UK at a price that is cost-effective and fair.”
The deal is confidential, but will probably result in the annual costs of the drug being reduced to a lot less than the £100,000 originally set by Vertex (to what Health Secretary Matt Hancock calls “a fair price”), and in addition, two other drugs will be provided for those who need them – Symkevi and Kalydeco. While the first is for patients 12 years old and over, the second can be used from 12 months of age. The agreement includes a full review of all drug data by NICE to ensure, among other things, its cost-effectiveness.
The deal was achieved with the active support of the Scottish government, which came to an agreement with Vertex a month ago. It also means great relief for thousands of patients and their families who had been campaigning in support of NHS England in its struggle to get the drug released for UK patients. Under the deal, Orkambi will also be available in Northern Ireland and Wales for the same price if the governments there so desire, though it is not known whether they will take advantage of this condition.
Cystic fibrosis
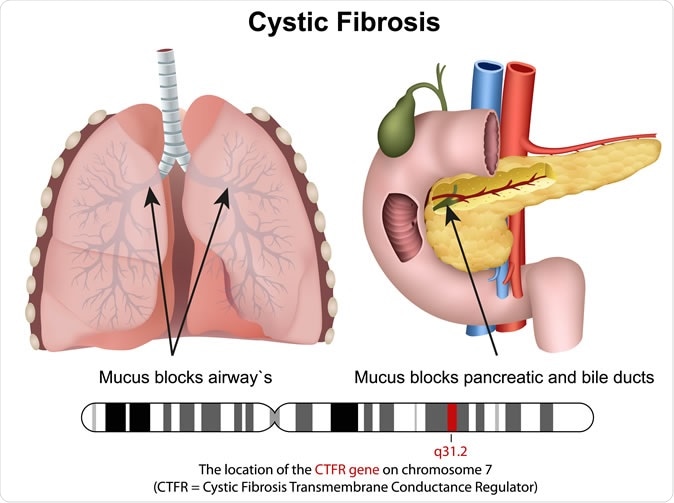
Cystic fibrosis is a genetic disease that is associated with the production of unduly thick and tenacious mucus from the airways, leading to chronic and intractable lung disease and premature death. Patients with cystic fibrosis die before they attain their 32nd birthday in about half of the cases. The mutations in the CFTR gene are responsible for the production of abnormal forms of the protein that helps to transport sodium, chloride and water in and out of the body cells – which is responsible for the excessively sticky mucus that clogs airways and the gut. CF affects about 30,000 patients in the US, and about 75,000 patients worldwide.
Patients and their families have been seeing the drug “dangling like a carrot” in front of their eyes for months, only to be denied access to it. Parents were unanimous in their delight, one mother (Christina Walker) calling it “the best [Christmas] present ever.” Simon Stevens, NHS chief executive, comments on the compromise as being “good for patients and fair to British taxpayers.” Vertex said they were glad “to get our medicines to cystic fibrosis patients as soon as possible.”
Cystic Fibrosis Trust spokesperson David Ramsden also chimed in with words of appreciation, saying, “This is a very special day… I want to thank people everyone who has been part of this campaign.”
How Orkambi works
Orkambi (a lumacaftor/ivacaftor combo) has been designed to counter the F508del mutation, which is by far the most common of CF-causing mutations in the CFTR gene. It is indicated for patients with two copies of this mutation, which means they have no working CFTR genes at all. It is thus estimated that it can be of use for about 50% of CF patients in the UK, who number about 10,000 in all. Lumacaftor is a corrector, and brings the defective chloride channel protein to the cell surface where they can be, while ivacaftor is a potentiator, and facilitates the opening of these channels so they can transport ions more effectively.
And now, Trikafta gains approval
Just a couple of days earlier, the US Food and Drug Administration (FDA) issued approval for another new drug, Trikafta, also made by Vertex. Trikafta is a combination of three drugs (Elexacaftor, tezacaftor and ivacaftor). The first two are correctors and the last a potentiator of the defective protein.
Trikafta is meant for patients who have at least one copy of the F508del mutation, and can be used in patients 12 years of age and older. It could thus benefit 90% of CF patients, the vast majority of whom have one or two copies of this mutation. However, it comes with a hefty price tag – well over $300,000 per patient per year. The treatment increased the lung capacity of CF patients by 14%, in clinical trials. The new slew of drugs thus offers more than a ray of hope to these patients.
Sources:
- Triggle, N. (2019). Cystic fibrosis drug given green light in England. https://www.bbc.com/news/health-50144742
- Bbc.com. (2018). Cystic fibrosis: Company urged to lower cost of life-changing drug. https://www.bbc.com/news/uk-43849586
- Roberts, L. (2019). Cystic fibrosis 'wonder drug' to be provided on the NHS after three year fight. https://www.telegraph.co.uk/news/2019/10/24/cystic-fibrosis-wonder-drug-provided-nhs-three-year-fight/
- Cff.org. (2019). Elexacaftor + tezacaftor + ivacaftor (Trikafta™). https://www.cff.org/Trials/Pipeline/details/10150/Elexacaftor-tezacaftor-ivacaftor-Trikafta






















.png)












No hay comentarios:
Publicar un comentario