Volume 25, Number 12—December 2019
Research
Epidemiologic, Entomologic, and Virologic Factors of the 2014–15 Ross River Virus Outbreak, Queensland, Australia
On This Page
Downloads
Article Metrics
Cassie C. Jansen, Martin A. Shivas, Fiona J. May, Alyssa T. Pyke, Michael B. Onn, Kerryn Lodo, Sonja Hall-Mendelin, Jamie L. McMahon, Brian L. Montgomery, Jonathan M. Darbro, Stephen L. Doggett, and Andrew F. van den Hurk
Abstract
Australia experienced its largest recorded outbreak of Ross River virus (RRV) during the 2014–15 reporting year, comprising >10,000 reported cases. We investigated epidemiologic, entomologic, and virologic factors that potentially contributed to the scale of the outbreak in Queensland, the state with the highest number of notifications (6,371). Spatial analysis of human cases showed that notifications were geographically widespread. In Brisbane, human case notifications and virus detections in mosquitoes occurred across inland and coastal locations. Viral sequence data demonstrated 2 RRV lineages (northeastern genotypes I and II) were circulating, and a new strain containing 3 unique amino acid changes in the envelope 2 protein was identified. Longitudinal mosquito collections demonstrated unusually high relative abundance of Culex annulirostris and Aedes procax mosquitoes, attributable to extensive freshwater larval habitats caused by early and persistent rainfall during the reporting year. Increased prevalence of these mosquitoes probably contributed to the scale of this outbreak.
Ross River virus (RRV; family Togaviridae, genus Alphavirus) is distributed throughout Australasia and has caused outbreaks involving thousands of humans in the western Pacific (1). RRV is the most commonly reported endemic arboviral infection in Australia; a mean of 4,541 cases/year were recorded during 2000–2016 (2). Cases are reported from every state and territory of Australia, and Queensland accounts for a large percentage (40%–65% during 2000–2006) (2).
Similar to the disease spectrum of related chikungunya virus, RRV infection causes polyarthritis and, in some cases, fever, maculopapular rash, fatigue, myalgia, lethargy, and headache (3,4). Many infections are asymptomatic and do not result in clinical disease (5), but debilitating arthritis of 3–6 months duration can occur in some patients (5–7). RRV ecology is complex, involving zoonotic transmission between multiple mosquitoes and vertebrates (8). Although numerous species may be hosts for RRV, the predominant vertebrate hosts are considered to be macropods (e.g., kangaroos and wallabies) (1,9,10). Humans have been implicated as hosts in outbreaks where macropods were absent (11,12). Overall, >40 mosquito species have yielded RRV isolates, although Aedes vigilax, Aedes camptorhynchus, and Culex annulirostris mosquitoes are considered the key vectors (13). Other species can be involved in specific locations (8,14), and transmission dynamics appear locally unique.
During the 2014–15 reporting year (i.e., July 1, 2014–June 30, 2015), a widespread RRV epidemic occurred in Australia; 10,074 cases were reported to the National Notifiable Diseases Surveillance System (15). This epidemic represented the highest number of RRV notifications ever reported in a season since 1993, when human RRV infection became nationally notifiable. In total, 63% (6,371) of notifications were from Queensland, Australia’s third-most populous state (15). We investigated the epidemiologic, entomologic, and virologic characteristics of the outbreak in Brisbane, the Queensland capital.
Study Area
Brisbane is situated at 27°28′S and 153°01′E on Australia’s eastern coast. The Brisbane local government area (LGA) comprises 1,367 km2 and, on June 30, 2015, had an estimated residential population of 1,165,437 (16). Brisbane has a subtropical climate (Köppen climate classification Cfa); monthly average temperatures range 10°C–22°C in winter and 20°C–29°C in summer. Approximately two thirds of the annual mean rainfall (1,149 mm) falls during November–March (17).
Human Case Notifications
The Queensland Notifiable Conditions Surveillance System (18) houses data on notifiable conditions in Queensland as outlined in the Public Health Act 2005 (19). We defined an RRV notification as the national case definition (i.e., a laboratory diagnosis of RRV) (20), but in 2016, this definition was changed to reduce the effect of false-positive notifications resulting from single IgM-positive test results. Thus, notifications reported herein might include false-positives. We assigned an LGA to notified cases using patient residential addresses. We extracted notification data, including date of specimen collection (used as a proxy for illness onset because this information was not systematically collected), residential address, and LGA, from the Notifiable Conditions Surveillance System for the period January 1, 1990–June 30, 2015. We present data as annual totals by reporting year, defined as July 1 of one year through June 30 of the next year, to reflect seasonality of mosquito abundance and mosquitoborne disease notifications and provide consistency with the national reporting convention. We numbered weeks as specified by ISO 8601:2004 (21), with week 1 starting on a Monday and containing the first Thursday of the calendar year.
We tabulated RRV notifications in the Brisbane LGA by week of specimen collection and Australian Statistical Geography Standard statistical area level 2 (22) and visualized using QGIS 2.18.1 (). Because location of exposures were unknown, we used patient residential address to map the spatial distribution of notifications. We performed all case data analyses in Stata SE 15 () and calculated rates (per 100,000 population) using estimated Queensland residential population data (23). We obtained ethics approval to conduct this research through the Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (reference no. HREC/15/QRCH/230).
Mosquito Collections
We collected mosquitoes weekly at 9 sites representing the larval habitat diversity of implicated RRV vectors and their proximity to human habitation. Trap sites varied by distance to larval habitats. Four sites were within 500 m of a saltmarsh, and 5 were close to freshwater habitats; some freshwater habitats were also near urban areas and considered suburban larval habitats (Table 1; Figure 1). We collected mosquitoes using PB light traps (Pacific BioLogics, ) baited with carbon dioxide (2-kg dry ice pellets) and 1-octen-3-ol (24) operated 4:00 PM –7:00 AM .
To account for occasional variation in the number of traps set (resulting from trap failures and prohibitive weather), for each week, we calculated the mean count of all mosquito species per trap and mean relative abundance of each mosquito species per trap. We compared the mean count of all mosquitoes per trap in the 2014–15 season with those of the other reporting years using Poisson regression. We compared the mean relative abundance of mosquito species comprising >5% of the total trap catch in 2014–15 with their mean relative abundances in the previous 2 reporting years using the 2-sample test of proportions. We considered p values <0.05 significant for all statistical tests.
For each week, we compared the number of human cases notified in the Brisbane LGA with the mean total mosquito count per trap and the mean relative abundance of frequently collected mosquitoes (i.e., those comprising >5% of the total trap catch in 2014–15) using Spearman rank correlation. We similarly compared the lag time of 0–8 weeks between mosquito counts and human case notifications.
Virus Detection in Mosquito Saliva and Mosquito Pools
We used 2 methods to acquire mosquito samples for RRV screening (Appendix). The first method was the sugar-based system described by Flies et al. (25), which involves collecting mosquito saliva expectorated during feedings (26). We deployed traps containing honey-soaked Flinders Technology Associates (FTA) cards (Whatman International Ltd, ) overnight on 15 occasions at weekly intervals during February 3–May 20, 2015 (weeks 6–21), excluding week 18. For the second method, we pooled whole mosquitoes collected in traps during February 3–March 10, 2015 (weeks 6–11), by species, trap, and trap night into groups of <100 mosquitoes.
We used a cell culture ELISA (27) to detect RRV in mosquito pools. We used an RRV-specific TaqMan real-time reverse transcription PCR (rRT-PCR) (28) to detect RRV RNA extracted from FTA cards and RRV RNA from mosquito pools acquired from traps that yielded RRV-positive FTA cards. We also performed rRT-PCR on mosquito samples derived from traps where a high level of mosquito death was observed during the 24-hour holding period after trap collection. Mosquito death compromises virus integrity and subsequent detection in the cell culture ELISA.
Sequence Analysis
We extracted virus RNA from patient serum samples, mosquito homogenates, FTA cards, and infected C6/36 cell culture supernatants. We amplified and sequenced the complete envelope (E) 3 and E2 gene regions (1,458 nt in total) using RRV-specific primers (Appendix Table 2) and 2 overlapping RT-PCR reactions (Appendix). We phylogenetically compared the RRV E3 and E2 sequences from samples collected in Brisbane during the 2014–15 outbreak with those of archived viruses from Brisbane and other locations around Australia isolated during 1959–2016 (Appendix Table 1).
Study Area Climate
The weather of southeast Queensland during the 2014–15 reporting year was characterized by early and consistent weekly rainfall from mid-November through late February (17), followed by drier weather interspersed with several large rain events. A total of 1,595 mm of rain fell, representing 152% of the Brisbane long-term average (Table 2). Of note, the preceding reporting year was unusually dry; only 55% of the long-term average rainfall fell.
Human Case Notifications
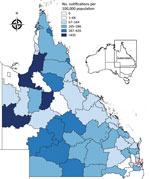
In the 2014–15 reporting year, 10,074 RRV notifications were reported nationally through the National Notifiable Diseases Surveillance System. The number of notifications in Queensland was 6,371, considerably higher than the mean of 1,854 cases reported annually over the previous 5 years and the largest number reported since statewide RRV surveillance began in 1990. Despite being the highest number of annual RRV notifications reported, the Queensland notification rate in 2014–15 (135 notifications/100,000 population) was lower than that in 1995–96 (150 notifications/100,000 population; Figure 2), a finding attributable to an increase in population over time. However, the mean rate for the 5 years before the 2014–15 outbreak was 41 notifications/100,000 population.
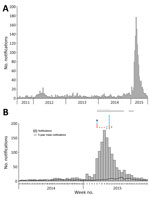
The 2014–15 notification rates varied by Queensland LGA (Figure 3). In total, 1,454 RRV notifications were reported in the Brisbane LGA in 2014–15. The number of weekly notifications first increased in Brisbane in week 2 of 2015 (25 cases; Figure 1, panel A; Figure 4). A marked increase occurred in week 6 (79 cases, compared with the average of 16.8 cases of the preceding 5 weeks), and the highest number occurred in week 9 (177 cases; Figure 1, panels B, C; Figure 4). The number of weekly case notifications returned to pre-outbreak levels by week 21 (Figure 4).
Notifications were widespread across Brisbane throughout the outbreak (Figure 1, panels A–D). No spatial clustering by statistical area level 2 was observed for notifications at any time during the outbreak.
Mosquito Collections
During 2014–15, a total of 411,328 mosquitoes (mean 877 mosquitoes per trap night) comprising >35 species were collected (Appendix Table 3). This number is a significant increase compared with the 204,220 (mean 498 mosquitoes/trap night) collected during the 2012–13 reporting year and 108,422 (mean 232 mosquitoes/trap night) collected during the 2013–14 reporting year (p<0.001). Ae. vigilax, Cx. annulirostris, and Aedes procax mosquitoes were the only species that comprised >5% of the trap catch during the 2014–15 reporting year. Ae. vigilax populations dominated collections in all years. Only Cx. annulirostris and Ae. procax mosquitoes significantly increased in abundance during the 2014–15 reporting year compared with previous reporting years (p<0.001). The relative abundance of all other species was not significantly increased in 2014–15 compared with previous years.
Cx. annulirostris populations accounted for 34% (140,287/411,328) of the total trap catch in 2014–15, a relative abundance significantly higher than those recorded for the 2012–13 (20%, 39,858/204,220; p<0.001) and 2013–14 (12%, 12,650/108,422; p<0.001) reporting years. During 2014–15, Cx. annulirostris mosquitoes showed an earlier than usual increase in abundance, and elevated counts were sustained throughout the outbreak (data not shown). The initial increase in weekly collections of this mosquito population observed starting week 50 of 2014 coincided with an increased number of weekly case notifications. The correlation between the mean relative abundance of Cx. annulirostris populations and RRV notifications was strong and significant (Spearman rank correlation coefficient ρ = 0.6190; p<0.001) only when a 3-week lag from mosquito abundance to human case notifications was applied. No correlation between the mean relative abundance of Cx. annulirostris mosquitoes and RRV notifications was observed in other reporting years (data not shown).
The Ae. procax population accounted for 6.4% (26,408/411,328) of the total trap catch in 2014–15, a relative abundance significantly higher than those recorded for the 2012–13 (2.3%, 4,654/204,220; p<0.001) and 2013–14 (1.4%, 1,570/108,422; p<0.001) reporting years. As with Cx. annulirostris mosquitoes, Ae. procax mosquito abundance increased starting week 50 of 2014 but did not reach a sustained peak until week 10 of 2015 and did not decrease until week 18 of 2015 (data not shown). As a result, Ae. procax mosquito mean relative abundance only moderately correlated with RRV notifications; a 2-week lag produced the highest correlation (ρ = 0.5543; p<0.001). No correlation was observed in any other reporting year (data not shown).
More Ae. vigilax mosquitoes were collected in 2014–15 than in other years. However, the relative abundance was only 51% (211,008/411,328) of the total trap catch, significantly lower than that of 2012–13 (60%, 123,024/204,220; p<0.001) and 2013–14 (78%, 84,133/108,422; p<0.001). Ae. vigilax mosquito numbers peaked in December 2014 (data not shown) but returned to typical numbers by early January, consistent with a weak negative correlation with RRV notifications (ρ = –0.3553, p = 0.009).
Virus Detection in Mosquito Pools and FTA Cards
A total of 135 honey-soaked FTA cards were deployed in mosquito traps during February 3–May 20, 2015, and we detected RRV RNA on 12 (8.9%) of them (Figure 4, panel B). On the first week of deployment (week 6 of 2015), 4 cards were positive for RRV RNA. Except for week 10, >1 card was positive each week during weeks 6–12, after which RRV was not detected. RRV was detected 3 times from FTA cards deployed March 11, 2015 (week 11), and 2 times from cards deployed March 18, 2015 (week 12; Figure 4, panel B). Except for Fig Tree Pocket, RRV RNA was detected >1 time from each trap location.
We processed 21,250 mosquitoes (5% of total collected in 2014–15), representing >20 species, for RRV detection (Appendix Table 4). Mosquitoes were combined into 385 pools and screened by cell culture ELISA. We also processed 155 pools, representing 10,112 mosquitoes, for rRT-PCR. A single pool of 68 Cx. annulirostris mosquitoes collected from Lota in week 6 of 2015 was positive by cell culture ELISA and rRT-PCR. One pool each of Ae. vigilax and Culex orbostiensis mosquitoes collected in the same trap on the same trap night as the Cx. annulirostris population were positive by rRT-PCR. RRV was also detected in a pool of 4 Mansonia uniformis mosquitoes collected in week 10 of 2015 at Hemmant. Viral RNA was detected in an additional 11 pools comprising mosquitoes from the trap deployed at Hemmant in weeks 10 (1 pool) and 11 (10 pools) of 2015. However, the species of mosquitoes in these pools could not be identified morphologically because of sample damage caused by permeation of rain into traps. Thus, the high number of RRV-positive pools from these traps could represent cross-contamination caused by parts of RRV-positive mosquitoes sticking to RRV-negative mosquitoes. Regardless, this data is evidence that RRV was present at Hemmant during these weeks.
Virus Nucleotide Sequence Phylogenetic Analysis
We determined the complete E3 and E2 gene sequences of 32 RRV samples and phylogenetically compared them with 9 additional RRV sequences from GenBank (accession nos. HM234643, M20162, GQ433354–60). The maximum-likelihood phylogenetic tree inferred from these sequences demonstrated all isolates belonged to the northeastern genotype (Figure 5). The 32 RRV sequences sampled over a 27-year period grouped within 1 of 2 major northeastern lineages, designated I and II (Figure 5). The phylogenetic groupings of BNE2015b (human origin, GenBank accession no. KX757013) and BNE-2885 (mosquito origin, GenBank accession no. KX757014) from Brisbane into lineage I and BNE2015a (human origin, accession no. KX757012) from Brisbane and 19661 (mosquito origin, accession no. KY290883) from Tweed, New South Wales, Australia, into lineage II demonstrate co-circulation of both lineages in southeast Queensland and northeast New South Wales during the 2014–15 outbreak.
Sequences of the outbreak isolates BNE2015b (lineage I) and BNE2015a (lineage II) were highly similar (98.4% nucleotide identity, 99.0% amino acid identity). Within lineage I, 2 sublineages (Ia and Ib) were demonstrated (Figure 5). In a comparison of amino acid sequences, except for the 203769 isolate (Queensland 2015) sequence, which was most similar to the LGRH-7021 (Longreach, Queensland, 2013) isolate sequence, the 2015 and 2016 sublineage Ib sequences all contained an A389T substitution within E2. Within lineage II, the E3 and E2 sequences of isolates 19661 (from an FTA card) and BNE2015a (from a patient) sampled during the 2014–15 outbreak shared 100% nucleotide and amino acid identities. Of note, these 2 sequences contained 3 unique amino acid substitutions in the E2 gene (A369T, M376I, T384A). Another unique E2 amino acid substitution, M45K, was demonstrated in 3 New South Wales 2015 RRV sequences obtained from mosquitoes (188448–50).
Outbreaks of RRV involving hundreds to thousands of cases have been reported from all mainland states of Australia (29). The 2014–15 outbreak was unprecedented in the high number of cases reported and large area of the eastern seaboard affected. Our investigations confirmed that human case notifications were distributed across the Brisbane LGA throughout the season, including before the outbreak, early in the outbreak, and at the peak of notifications. The concurrent detection of virus from mosquitoes across Brisbane provides compelling evidence that RRV activity was widespread and the exposure risk for humans high across all suburbs and districts. We suggest that a combination of ecologic factors contributed to the magnitude of the RRV outbreak in Brisbane in 2014–15.
Previous RRV outbreaks in Australia were preceded by above-average rainfall (29,30). The weather in Brisbane during 2014–15 was unusual, characterized by early elevated rainfall that persisted throughout the summer and resulted in total rainfall exceeding the historical mean. These conditions provided temporary freshwater larval habitats for many mosquito species, including Cx. annulirostris populations, for an unusually long period. The early increase in Cx. annulirostris abundance, which remained high, coupled with a correlation with RRV notifications suggest that this species was a key vector during the outbreak. In addition, the widespread geographic distribution of Cx. annulirostris mosquitoes (data not shown), which reflected the distribution of human notifications, further supports the involvement of this species in the outbreak. The Cx. annulirostris mosquito is a competent laboratory vector of RRV that has yielded numerous field isolates in previous studies (31,32) and yielded field isolates in our study. Furthermore, evidence has implicated Cx. annulirostris mosquito involvement in RRV outbreaks in New South Wales in 2014–15 (33) and New South Wales and Victoria in 2016–17 (34,35).
On the basis of their temporal and spatial abundance, Ae. procax mosquitoes also showed a moderate correlation with human RRV notifications in 2014–15, albeit at a lower relative abundance than Cx. annulirostris mosquitoes. Although RRV was not detected in the Ae. procax populations herein, this species has previously yielded relatively high numbers of field isolates (when compared with the number of specimens tested) and demonstrates high vector competence for RRV in the laboratory (32). Like Cx. annulirostris mosquitoes, Ae. procax mosquitoes feed on a range of mammals (36), so they might play a greater role in urban transmission of arboviruses than previously considered (32,37,38).
The most abundant saltmarsh mosquito in southeast Queensland, Ae. vigilax, reached notably high numbers in 2014–15. However, this mosquito’s relative abundance was significantly lower in 2014–15 than in previous years. Furthermore, the temporal abundance of Ae. vigilax populations peaked earlier and had a weak and negative correlation with human case notifications, suggesting that even if involved in enzootic transmission this species was unlikely responsible for sustained transmission to humans throughout the outbreak. In addition, in previous years, high numbers of Ae. vigilax mosquitoes were present in the Brisbane LGA without increased numbers of RRV notifications (e.g., 2012–13 and 2013–14), and low numbers were present in years when RRV notifications were above average (e.g., 2011–12).
Given the complexity of RRV transmission cycles, the role of other common species should not be discounted. Of the remaining 2 species from which RRV was detected during this study, Ma. uniformis mosquitoes have previously yielded isolates and been shown to transmit the virus in laboratory experiments (32). In contrast, RRV has not been detected in Cx. orbostiensis mosquitoes previously, despite extensive testing for field isolates in New South Wales since 1988, so its status as an RRV vector is unknown.
In Australia, RRV comprises 3 distinct genotypes, western, northeastern, and southeastern, named for the location in which they predominate (39,40). The finding of northeastern genotype lineage I and II sequences in human and mosquito samples suggests both lineages contributed to the 2014–15 outbreak and confirms their persistent transmission in eastern Australia. Our results are consistent with previous studies suggesting that the distribution of lineages I and II in eastern Australia are not constrained by geographic distance or location.
We detected several amino acid substitutions in E2 of most 2015 and 2016 RRV isolates, including 3 (A369T, M376I, T384A) in a strain represented by isolates 19661 and BNE2015a. Of note, A369T, M376I, T384A, and A389T all occurred within the putative E2 C-terminal anchor sequence comprising amino acids 365–90 (41). Whether these amino acid changes are pleiotropic or represent adaptive changes related to the interaction of E2 with E1 or other structural proteins during viral assembly is unknown.
We investigated entomologic, epidemiologic, and virologic factors associated with the 2014–15 RRV outbreak in Brisbane. A missing factor in the investigation of this and previous outbreaks is the contribution of nonhuman hosts to epidemic transmission. Numerous vertebrate species are likely involved in RRV maintenance (10), and the role of each species during outbreaks is probably complex. The widespread distribution of RRV during 2014–15 suggests the involvement of a common ubiquitous species or several reservoir species. Furthermore, limited RRV activity in the preceding years might have increased the pool of nonimmune hosts, contributing to the scale of the outbreak.
Overall, early and consistent rainfall in 2014–15 in southeast Queensland probably contributed to a high abundance and the survival of adult mosquitoes, providing ideal conditions for the largest recorded outbreak of RRV. As demonstrated by the spatial distribution of RRV patients and virus detections in mosquitoes, virus activity was widespread across the Brisbane LGA. Notwithstanding the potential role of other mosquito species in ongoing transmission of RRV, we propose that freshwater species (particularly Cx. annulirostris and Ae. procax mosquitoes) were likely key drivers of the outbreak activity in Brisbane in 2014–15. We demonstrate that the risk for RRV infection in humans is widespread and driven by complex factors in Queensland.
Dr. Jansen is the state consultant medical entomologist for the Communicable Diseases Branch of the Department of Health, Queensland Government, Australia. Dr. Jansen’s research interests span various aspects of medical entomology, including the development of preparedness and mitigation strategies for exotic arboviruses and invasive mosquitoes and the description of transmission cycles of endemic and exotic arboviruses.
Acknowledgments
We acknowledge Ian Myles for assistance with field deployment of mosquito traps and Mike Muller and Cameron Webb for kindly providing technical advice on entomologic aspects of the study. We thank Glen Hewitson, Doris Genge, and Jane Cameron for assistance with molecular analysis; Bruce Harrower, Peter Burtonclay, and Tanya Constantino for cell culture maintenance; and Frederick Moore for laboratory management. Mohana Rajmokan is acknowledged for epidemiologic data management. Bob Gibb generously provided virus samples for analysis.
This study was partially funded by the Mosquito and Arbovirus Research Committee and the Queensland Health Forensic and Scientific Services.
References
- van den Hurk AF, Jansen CC. Arboviruses of Oceania. In: Loukas A, editor. Neglected tropical diseases—Oceania. Cham (Switzerland): Springer International Publishing; 2016. p. 193–235.
- Australian Government Department of Health. Introduction to the National Notifiable Diseases Surveillance System. 2015 Jun 9 [cited 2017 Oct 23].
- Fraser JR. Epidemic polyarthritis and Ross River virus disease. Clin Rheum Dis. 1986;12:369–88.
- Flexman JP, Smith DW, Mackenzie JS, Fraser JRE, Bass SP, Hueston L, et al. A comparison of the diseases caused by Ross River virus and Barmah Forest virus. Med J Aust. 1998;169:159–63.
- Harley D, Sleigh A, Ritchie S. Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin Microbiol Rev. 2001;14:909–32.
- Harley D, Bossingham D, Purdie DM, Pandeya N, Sleigh AC. Ross River virus disease in tropical Queensland: evolution of rheumatic manifestations in an inception cohort followed for six months. Med J Aust. 2002;177:352–5.
- Mylonas AD, Brown AM, Carthew TL, McGrath B, Purdie DM, Pandeya N, et al. Natural history of Ross River virus-induced epidemic polyarthritis. Med J Aust. 2002;177:356–60.
- Claflin SB, Webb CE. Ross River virus: many vectors and unusual hosts make for an unpredictable pathogen. PLoS Pathog. 2015;11:
e1005070 . - Kay BH, Hall RA, Fanning ID, Mottram P, Young PL, Pollitt CC. Experimental infection of vertebrates with Murray Valley encephalitis and Ross River viruses. Arbovirus Res Aust. 1986;4:71–5.
- Stephenson EB, Peel AJ, Reid SA, Jansen CC, McCallum H. The non-human reservoirs of Ross River virus: a systematic review of the evidence. Parasit Vectors. 2018;11:188.
- Tesh RB, McLean RG, Shroyer DA, Calisher CH, Rosen L. Ross River virus (Togaviridae: Alphavirus) infection (epidemic polyarthritis) in American Samoa. Trans R Soc Trop Med Hyg. 1981;75:426–31.
- Lindsay MD, Johansen C, Broom AK, D’Ercole M, Wright AE, Condon R, et al. The epidemiology of outbreaks of Ross River virus infection in Western Australia in 1991–1992. Arbovirus Res Aust. 1993;6:72–6.
- Russell RC. Ross River virus: ecology and distribution. Annu Rev Entomol. 2002;47:1–31.
- Harley D, Ritchie S, Phillips D, van den Hurk A. Mosquito isolates of Ross River virus from Cairns, Queensland, Australia. Am J Trop Med Hyg. 2000;62:561–5.
- Knope K, Doggett SL, Jansen CC, Johansen CA, Kurucz N, Feldman R, et al.; the National Arbovirus and Malaria Advisory Committee. Arboviral diseases and malaria in Australia, 2014–15: annual report of the National Arbovirus and Malaria Advisory Committee. Commun Dis Intell (2018). 2019;43.
- Queensland Treasury. Population estimate. Regions. Estimated resident population by local government area (LGA), Queensland, 1991 to 2018. 2019 Mar 29 [cited 2019 Oct 15].
- Australian Government Bureau of Meteorology. Climate statistics for Australian locations. Monthly climate statistics. 2017 [cited 2017 Jun 13].
- Queensland Government Department of Health. Notifiable conditions register. 2016 Feb 3 [cited 2017 Oct 23].
- Queensland Health. Public Health Act 2005. 2019 Oct 11 [cited 2019 Oct 15].
- Australian Government Department of Health. Ross River virus infection case definition. Australian national notifiable diseases case definitions. 2016 Jan 1 [cited 2019 Oct 15].
- International Organization for Standardization. ISO 8601:2004. Data elements and interchange formats—information interchange—representation of dates and times. 2004 Dec [cited 2017 Oct 23].
- Australian Bureau of Statistics. 1270.0.55.001-Australian Statistical Geography Standard (ASGS): volume 1-main structure and greater capital city statistical areas, July 2011. 2016 Jul 11 [cited 2017 Oct 23].
- Australian Bureau of Statistics. Australian Demographic Statistics Mar 2017. 2017 Dec 13 [cited 2017 Dec 23].
- Van Essen PH, Kemme JA, Ritchie SA, Kay BH. Differential responses of Aedes and Culex mosquitoes to octenol or light in combination with carbon dioxide in Queensland, Australia. Med Vet Entomol. 1994;8:63–7.
- Flies EJ, Toi C, Weinstein P, Doggett SL, Williams CR. Converting mosquito surveillance to arbovirus surveillance with honey-baited nucleic acid preservation cards. Vector Borne Zoonotic Dis. 2015;15:397–403.
- Hall-Mendelin S, Ritchie SA, Johansen CA, Zborowski P, Cortis G, Dandridge S, et al. Exploiting mosquito sugar feeding to detect mosquito-borne pathogens. Proc Natl Acad Sci U S A. 2010;107:11255–9.
- Broom AK, Hall RA, Johansen CA, Oliveira N, Howard MA, Lindsay MD, et al. Identification of Australian arboviruses in inoculated cell cultures using monoclonal antibodies in ELISA. Pathology. 1998;30:286–8.
- Hall RA, Prow NA, Pyke AT. Ross River virus. In: Liu D, editor. Molecular detection of human viral pathogens. Boca Raton (FL): CRC Press; 2011. p. 349–59.
- Kelly-Hope LA, Purdie DM, Kay BH. Ross River virus disease in Australia, 1886-1998, with analysis of risk factors associated with outbreaks. J Med Entomol. 2004;41:133–50.
- Woodruff RE, Guest CS, Garner MG, Becker N, Lindesay J, Carvan T, et al. Predicting Ross River virus epidemics from regional weather data. Epidemiology. 2002;13:384–93.
- Ritchie SA, Fanning ID, Phillips DA, Standfast HA, McGinn D, Kay BH. Ross River virus in mosquitoes (Diptera:Culicidae) during the 1994 epidemic around Brisbane, Australia. J Med Entomol. 1997;34:156–9.
- Ryan PA, Do KA, Kay BH. Definition of Ross River virus vectors at Maroochy Shire, Australia. J Med Entomol. 2000;37:146–52.
- Doggett S, Haniotis J, Clancy J, Webb C, Toi C, Hueston L, et al. The New South Wales Arbovirus Surveillance and Mosquito Monitoring Program, 2014–2015 annual report. 2015 [cited 2019 Oct 15].
- Wong S, Brown K, Crowder J, Chea S, Mee P, Batovska J, et al. Victorian Arbovirus Disease Control Program annual report 2016–2017. Melbourne (VIC, Australia): Agriculture Victoria; 2017.
- Doggett S, Haniotis J, Clancy J, Webb C, Toi C, Hueston L, et al. The New South Wales Arbovirus Surveillance and Mosquito Monitoring Program, 2016–2017 annual report. 2017 [cited 2019 Oct 15].
- Kay BH, Boyd AM, Ryan PA, Hall RA. Mosquito feeding patterns and natural infection of vertebrates with Ross River and Barmah Forest viruses in Brisbane, Australia. Am J Trop Med Hyg. 2007;76:417–23.
- Doggett SL, Clancy J, Haniotis J, Webb CE, Hueston L, Marchetti M, et al. Arbovirus and vector surveillance in New South Wales, 2004/5–2007/8. Arbovirus Res Aust. 2009;10:28–37.
- Webb CE, Jansen CC, van den Hurk AF, Russell RC. Is Aedes procax (Skuse) emerging as an important vector of arboviruses in coastal NSW? Arbovirus Res Aust. 2009;10:182.
- Sammels LM, Coelen RJ, Lindsay MD, Mackenzie JS. Geographic distribution and evolution of Ross River virus in Australia and the Pacific Islands. Virology. 1995;212:20–9.
- Jones A, Lowry K, Aaskov J, Holmes EC, Kitchen A. Molecular evolutionary dynamics of Ross River virus and implications for vaccine efficacy. J Gen Virol. 2010;91:182–8.
- Strauss EG, Lenches EM, Strauss JH. Molecular genetic evidence that the hydrophobic anchors of glycoproteins E2 and E1 interact during assembly of alphaviruses. J Virol. 2002;76:10188–94.
Figures
Tables
Suggested citation for this article: Jansen CC, Shivas MA, May FJ, Pyke AT, Onn MB, Lodo K, et al. Epidemiologic, entomologic, and virologic factors of the 2014–15 Ross River virus outbreak, Queensland, Australia. Emerg Infect Dis. 2019 Dec [date cited]. https://doi.org/10.3201/eid2512.181810
Original Publication Date: 10/28/2019
























.png)












No hay comentarios:
Publicar un comentario