Memory foam for vascular treatment receives FDA clearance
Shape memory foam embolization system deployed in Europe now cleared for U.S. markets
Shape Memory Medical recently announced FDA clearance for U.S. marketing of their IMPEDE Embolization Plug, a technology funded by the National Institute of Biomedical Imaging and Bioengineering(NIBIB) and created to block irregular blood vessels.
The clearance allows the revolutionary shape memory polymer technology to be used to improve a variety of vascular complications.
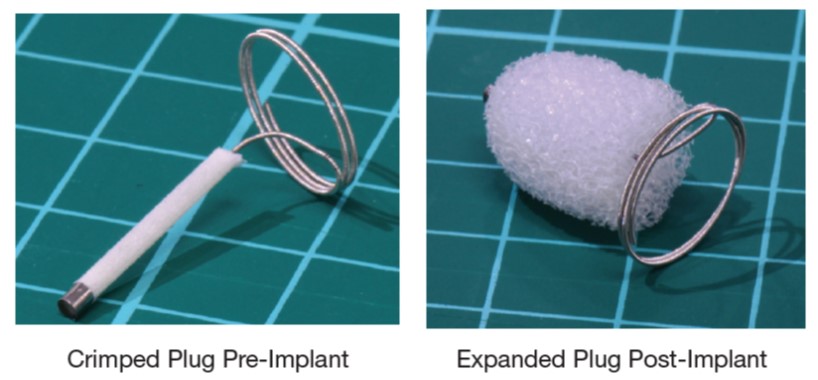
Left: Compressed shape memory polymer foam on catheter that is thread through blood vessels to treatment site. Right: Foam size and shape when fully expanded at treatment site to induce stable clot formation. Credit: Shape Memory Medical.


































No hay comentarios:
Publicar un comentario