
CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers
For years, the foundations of cancer treatment were surgery, chemotherapy, and radiation therapy. Over the last two decades, targeted therapies like imatinib (Gleevec®)and trastuzumab (Herceptin®)—drugs that target cancer cells by homing in on specific molecular changes seen primarily in those cells—have also cemented themselves as standard treatments for many cancers.
But over the past several years, immunotherapy—therapies that enlist and strengthen the power of a patient’s immune system to attack tumors—has emerged as what many in the cancer community now call the “fifth pillar” of cancer treatment.
A rapidly emerging immunotherapy approach is called adoptive cell transfer (ACT): collecting and using patients’ own immune cells to treat their cancer. There are several types of ACT (see “ACT: TILs, TCRs, and CARs”), but, thus far, the one that has advanced the furthest in clinical development is called CAR T-cell therapy.
Until recently, the use of CAR T-cell therapy has been restricted to small clinical trials, largely in patients with advanced blood cancers. But these treatments have nevertheless captured the attention of researchers and the public alike because of the remarkable responses they have produced in some patients—both children and adults—for whom all other treatments had stopped working.
In 2017, two CAR T-cell therapies were approved by the Food and Drug Administration (FDA), one for the treatment of children with acute lymphoblastic leukemia (ALL) and the other for adults with advanced lymphomas. Nevertheless, researchers caution that, in many respects, it’s still early days for CAR T cells and other forms of ACT, including questions about whether they will ever be effective against solid tumors like breast and colorectal cancer.
The different forms of ACT “are still being developed,” said Steven Rosenberg, M.D., Ph.D., chief of the Surgery Branch in NCI’s Center for Cancer Research (CCR), an immunotherapy pioneer whose lab was the first to report successful cancer treatment with CAR T cells.
But after several decades of painstaking research, the field has reached a tipping point, Dr. Rosenberg continued. In just the last few years, progress with CAR T cells and other ACT approaches has greatly accelerated, with researchers developing a better understanding of how these therapies work in patients and translating that knowledge into improvements in how they are developed and tested.
“In the next few years,” he said, “I think we’re going to see dramatic progress and push the boundaries of what many people thought was possible with these adoptive cell transfer–based treatments.”
A “Living Drug”
CAR T cells are the equivalent of “giving patients a living drug,” explained Renier J. Brentjens, M.D., Ph.D., of Memorial Sloan Kettering Cancer Center in New York, another early leader in the CAR T-cell field.
As its name implies, the backbone of CAR T-cell therapy is T cells, which are often called the workhorses of the immune system because of their critical role in orchestrating the immune response and killing cells infected by pathogens. The therapy requires drawing blood from patients and separating out the T cells. Next, using a disarmed virus, the T cells are genetically engineered to produce receptors on their surface called chimeric antigen receptors, or CARs.
These receptors are “synthetic molecules, they don’t exist naturally,” explained Carl June, M.D., of the University of Pennsylvania Abramson Cancer Center, during a recent presentation on CAR T cells at the National Institutes of Health campus. Dr. June has led a series of CAR T cell clinical trials, largely in patients with leukemia.
These special receptors allow the T cells to recognize and attach to a specific protein, or antigen, on tumor cells. The CAR T cell therapies furthest along in development target an antigen found on B cells called CD19 (see “The Making of a CAR T Cell”).
Once the collected T cells have been engineered to express the antigen-specific CAR, they are “expanded” in the laboratory into the hundreds of millions.
The final step is the infusion of the CAR T cells into the patient (which is preceded by a “lymphodepleting” chemotherapy regimen). If all goes as planned, the engineered cells further multiply in the patient’s body and, with guidance from their engineered receptor, recognize and kill cancer cells that harbor the antigen on their surfaces.
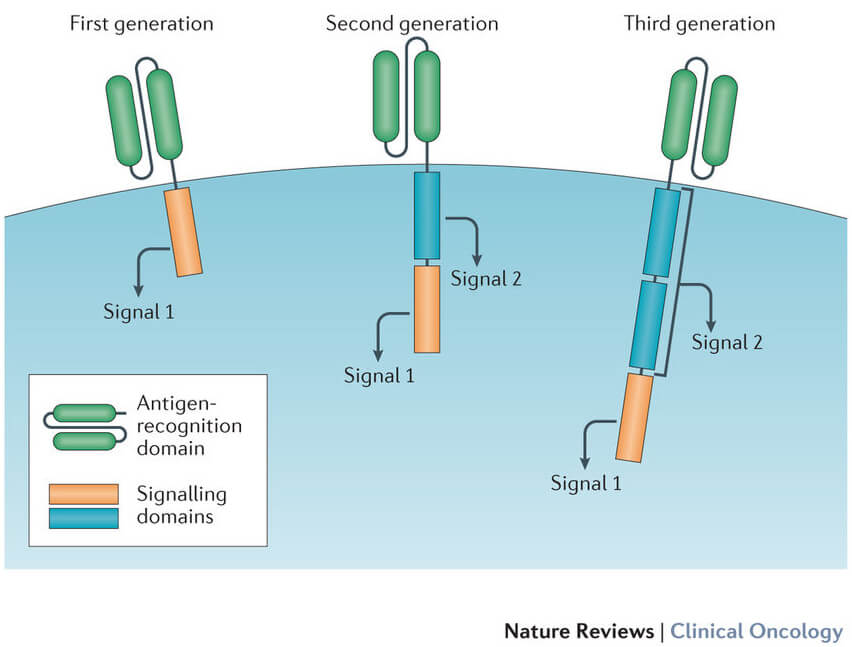
The Making of a CAR T Cell
A growing number of CAR T-cell therapies are being developed and tested in clinical studies.
Although there are important differences between these therapies, they all share similar components. The CAR on the cell’s surface is composed of fragments, or domains, of synthetic antibodies. The domains that are used can affect how well the receptor recognizes or binds to the antigen on the tumor cell.
The receptors rely on stimulation signals from inside the cell to do their job. So each CAR T cell has signaling and “co-stimulatory” domains inside the cell that signal the cell from the surface receptor. The different domains that are used can affect the cells’ overall function.
Over time, advances in the intracellular engineering of CAR T cells have improved the engineered T cells’ ability to produce more T cells after infusion into the patient (expansion) and survive longer in the circulation (persistence).
Advances have also been made in how long it takes to produce a batch of CAR T cells. Although it initially took several weeks, many labs have now reduced the time to less than 7 days.
A Possible Option Where None Had Existed
The initial development of CAR T-cell therapies has focused largely on ALL, the most common cancer in children.
More than 80% of children diagnosed with ALL that arises in B cells—the predominant type of pediatric ALL—will be cured by intensive chemotherapy. But for patients whose cancers return after chemotherapy or a stem cell transplant, the treatment options are “close to none,” said Stephan Grupp, M.D., Ph.D., of the Children’s Hospital of Philadelphia (CHOP).
Relapsed ALL, in fact, is a leading cause of death from childhood cancer.
Dr. Grupp has led several trials of CAR T cells in children and young adults with ALL that had recurred or was not responding to existing therapies. In one of these earlier trials, which used CD19-targeted CAR T cells, all signs of cancer disappeared (a complete response) in 27 of the 30 patients treated in the study, with many of these patients continuing to show no signs of recurrence long after the treatment.
These early successes laid the foundation for a larger trial of a CD19-targeted CAR T-cell therapy, called tisagenlecleucel (Kymriah™), for children and adolescents with ALL. Many of the patients who participated in the trial, funded by Novartis, had complete and long-lasting remissions. Based on the trial results, FDA approved tisagenlecleucel in August 2017.
Similar results have been seen in trials of CD19-targeted CAR T cells led by researchers in CCR’s Pediatric Oncology Branch (POB).
The progress made with CAR T-cell therapy in children with ALL “has been fantastic,” said Terry Fry, M.D., a lead investigator on several POB trials of CAR T cells. CD19-targeted CAR T cells were initially tested in adults. But the fact that the first approval is for a therapy for children and adolescents with ALL is a watershed moment, Dr. Fry continued.
The agency approving a new therapy in children before adults “is almost unheard of in cancer,” he said.
However, there is no shortage of promising data on CAR T cells used to treat adult patients with blood cancers. CD19-targeted CAR T cells have produced strong results not only in patients with ALL but also in patients with lymphomas. For example, in a small NCI-led trialof CAR T cells primarily in patients with advanced diffuse large B-cell lymphoma, more than half had complete responses to the treatment.
“Our data provide the first true glimpse of the potential of this approach in patients with aggressive lymphomas, who, until this point, were virtually untreatable,” said the trial’s lead investigator, James Kochenderfer, M.D., of the NCI Experimental Transplantation and Immunology Branch.
Since that time, findings from a larger trial funded by Kite Pharmaceuticals (which has a research agreement with NCI to develop ACT-based therapies) have confirmed these earlier results and formed the basis for FDA's approval of Kite’s CAR T-cell product, axicabtagene ciloleucel (Yescarta™), for some patients with lymphoma.
The results in lymphoma to date “have been incredibly successful,” Dr. Kochenderfer said, “and CAR T cells are almost certain to become a frequently-used therapy for several types of lymphoma.”
The rapid advances in and growth of CAR T-cell therapy has exceeded the expectations of even those who were early believers in its potential.
“Did I think it could work? Yes,” Dr. Brentjens said. But he initially thought it would be a “boutique therapy” limited to a very small, defined patient group. The experience over the past 5 years, including the entry of the biopharmaceutical industry into the field, has altered his outlook.
“We have cohorts of patients who would have been considered terminal who are now in durable and meaningful remissions with good quality of life for up to 5 years,” he continued. “So the enthusiasm for this technology is now quite high.”
Understanding, Managing Side Effects
Like all cancer therapies, CAR T-cell therapy can cause several worrisome, and sometimes fatal, side effects. One of the most frequent is cytokine release syndrome (CRS).
As part of their immune-related duties, T cells release cytokines, chemical messengers that help to stimulate and direct the immune response. In the case of CRS, there is a rapid and massive release of cytokines into the bloodstream, which can lead to dangerously high fevers and precipitous drops in blood pressure.
Ironically, CRS is considered an “on-target” effect of CAR T-cell therapy—that is, its presence demonstrates that active T cells are at work in the body. Generally, patients with the most extensive disease prior to receiving CAR T cells are more likely to experience severe CRS, Dr. Kochenderfer explained.
In many patients, both children and adults, CRS can be managed with standard supportive therapies, including steroids. And as researchers have gained more experience with CAR T-cell therapy, they’ve learned how to better manage the more serious cases of CRS.
Several years ago, for instance, the research team at CHOP noticed that patients experiencing severe CRS all had particularly high levels of IL-6, a cytokine that is secreted by T cells and macrophages in response to inflammation. So they turned to therapies that are approved to treat inflammatory conditions like juvenile arthritis, including the drug tocilizumab (Actemra®), which blocks IL-6 activity.
The approach worked, rapidly resolving the problem in most patients. Since that time, tocilizumab has become a standard therapy for managing severe CRS.
“We’ve learned how to grade [CRS], we’ve learned how to treat it,” Dr. Grupp said during an FDA advisory committee meeting on Novartis’ CD19-targeted therapy. “And IL-6 blockade was really the key.”
Another potential side effect of CAR T-cell therapy—an off-target effect—is a mass die off of B cells, known as B-cell aplasia. CD19 is also expressed on normal B cells, which are responsible for producing antibodies that kill pathogens. These normal B cells are also often killed by the infused CAR T cells. To compensate, many patients must receive immunoglobulin therapy, which provides them with the necessary antibodies to fight off infections.
More recently, another serious and potentially fatal side effect—swelling in the brain, or cerebral edema—has been seen in some of the larger trials being conducted to support potential FDA approval of CAR T-cell therapies for patients with advanced leukemias. One company, in fact, decided to halt further development of their leading CAR T-cell therapy after several patients in clinical trials died as a result of treatment-induced cerebral edema.
However, the problem appears to be limited, with the leaders of other trials of CAR T-cell therapies reporting no instances of cerebral edema.
Other so-called neurotoxicities—such as confusion or seizure-like activity—have been seen in most CAR T-cell therapy trials. But in nearly all patients the problem is short lived and reversible, Dr. Brentjens said.
There was speculation early on that these neurotoxicities might be related to CRS. But although researchers are still trying to get their hands around the mechanisms, he added, “I think most investigators [in the field] would agree that they’re distinct from CRS.”
New Target Antigens for CAR T Cells
Research on CAR T cells is continuing at a swift pace, mostly in patients with blood cancers, but also in patients with solid tumors. As the biopharmaceutical industry has become more involved in the field, for instance, the number of clinical trials testing CAR T cells has expanded dramatically, from just a handful 5 years ago to more than 180 and counting.
Most of the trials conducted to date have used CD19-targeted CAR T cells. But that’s changing quickly, in part out of necessity.
Some patients with ALL, for example, don’t respond to the CD19-targeted therapy. And even in those who experience a complete response, up to a third will see their disease return within a year, Dr. Fry said. Many of these disease recurrences have been linked to ALL cells’ no longer expressing CD19, a phenomenon known as antigen loss.
So, in children and young adults with advanced ALL, researchers in NCI’s POB are testing CAR T cells that target the CD22 protein, which is also often overexpressed by ALL cells. In the first trial of CD22-targeted CAR T cells, most treated patients had complete remissions, including patients whose cancer had progressed after initially having a complete response to CD19-targeted therapy.
Similar to the case with the CD19-targeted CAR T cells, however, relapses after CD22-targeted treatment are not uncommon, Dr. Fry explained.
“There is definitely room to improve from the standpoint of the durability of remissions,” he said.
One potential way to improve durability and perhaps at least forestall antigen loss, if not prevent it altogether, is to attack multiple antigens simultaneously. Several research groups, for example, are testing T cells that target both CD19 and CD22 in early-phase clinical trials.
CHOP researchers are also testing a CAR T cell that targets both CD19 and CD123, another antigen commonly found on leukemia cells. Early studies in animal models have suggested that this dual targeting may prevent antigen loss.
Antigen targets for CAR T-cell therapy have been identified in other blood cancers as well, including multiple myeloma.
Dr. Kochenderfer and his colleagues at NCI, as part of the collaboration with Kite, have developed CAR T cells that target the BCMA protein, which is found on nearly all myeloma cells.
In an early-phase clinical trial of BCMA-targeted CAR T cells in patients with advanced multiple myeloma, more than half of the patients had a complete response to the treatment. Kite has now launched a trial to test the BCMA-targeted T cells in a larger group of patients.
Expanding CAR T Cells to Solid Tumors?
There is some skepticism that CAR T cells will have the same success in solid tumors. Dr. Rosenberg believes that finding suitable antigens to target on solid tumors—which has been a major challenge—may prove to be too difficult in most cases.
“Efforts to identify unique antigens on the surface of solid tumors have largely been unsuccessful,” he said.
Researchers estimate that the overwhelming majority of tumor antigens reside inside tumor cells, out of the reach of CARs, which can only bind to antigens on the cell surface.
As a result, as has already been shown in melanoma, Dr. Rosenberg said that he believes other forms of ACT may be better suited for solid tumors.
But that doesn’t mean that researchers aren’t trying with CAR T cells.
For example, investigators are conducting trials of CAR T cells that target the protein mesothelin, which is overexpressed on tumor cells in some of the most deadly cancers, including pancreatic and lung cancers, and the protein EGFRvIII, which is present on nearly all tumor cells in patients with the aggressive brain cancer glioblastoma.
Early reports from these trials, however, have not reported the same success that’s been seen with blood cancers.
“As far as targeting antigens on solid tumors the same way we go after CD19, I don’t think that’s going to work in most cases,” Dr. Brentjens acknowledged.
Another key obstacle with solid tumors, he explained, is that components of the microenvironment that surrounds them conspire to blunt the immune response.
So success against solid tumors may require a “super T cell,” he said, that has been engineered to overcome the immune-suppressing environment of many advanced solid tumors. Work on a CAR T cell with these properties—an “armored” CAR T cell—is ongoing at Memorial Sloan Kettering, he said.
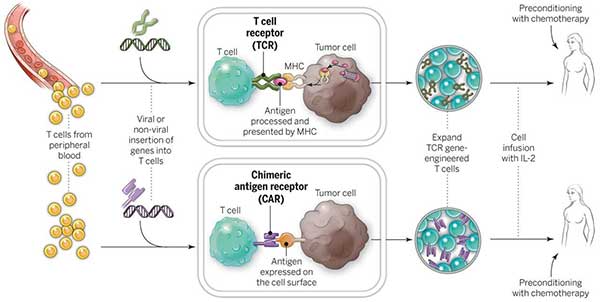
ACT: TILs, TCRs, and CARs
CAR T cells have garnered the lion’s share of the attention when it comes to the cellular therapies that fall under the ACT umbrella. But other forms of ACT have also shown promise in small clinical trials, including in patients with solid tumors.
One approach uses immune cells that have penetrated the environment in and around the tumor, known as tumor-infiltrating lymphocytes (TILs). Researchers at NCI were the first to use TILs to successfully treat patients with advanced cancer—initially in melanoma and later in several other cancers, including cervical cancer. More recently, NCI researchers have developed a technique for identifying TILs that recognize cancer cells with mutations specific to that cancer. In several cases, this approach has led to tumor regressions in patients with advanced colorectal and liver cancer.
The other primary approach to ACT involves engineering patients’ T cells to express a specific T-cell receptor (TCR). CARs use portions of synthetic antibodies that can recognize specific antigens only on the surface of cells. TCRs, on the other hand, use naturally occurring receptors that can also recognize antigens that are inside tumor cells. Small pieces of these antigens are shuttled to the cell surface and “presented” to the immune system as part of a collection of proteins called the MHC complex.
To date, TCR T cells have been tested in patients with a variety of solid tumors, showing promise in melanoma and sarcoma.
Evolution of CAR T-Cell Therapies
Other refinements or reconfigurations of CAR T cells are being tested. One approach is the development of CAR T-cell therapies that use immune cells collected not from patients, but from healthy donors. The idea is to create so-called off-the-shelf CAR T-cell therapies that are immediately available for use and don’t have to be manufactured for each patient.
The French company Cellectis, in fact, has launched a phase I trial of its off-the-shelf CD19-targeted CAR T-cell product in the United States for patients with advanced acute myeloid leukemia. The company’s product—which is made using a gene-editing technology known as TALEN—has already been tested in Europe, including in two infants with ALL who had exhausted all other treatment options. In both cases, the treatment was effective.
Numerous other approaches are under investigation. Researchers, for example, are using nanotechnology to create CAR T cells inside the body, developing CAR T cells with “off switches” as a means of preventing or limiting side effects like CRS, and using the gene-editing technology CRISPR/Cas9 to more precisely engineer the T cells.
But there is still more to do with existing CAR T-cell therapies, Dr. Fry said.
He is particularly enthusiastic about the potential to use CAR T cells earlier in the treatment process for children with ALL, specifically those who are at high risk (based on specific clinical factors) of their disease returning after their initial chemotherapy, which typically is given for approximately 2 and a half years.
In this scenario, he explained, if early indicators suggested that these high-risk patients weren’t having an optimal response to chemotherapy, it could be stopped and the patients could be treated with CAR T cells.
For patients who respond well, “they could be spared 2 more years of chemotherapy,” Dr. Fry said. “That’s amazing to think about.”



































No hay comentarios:
Publicar un comentario