
Volume 23, Number 4—April 2017
Research Letter
mcr-1 in Enterobacteriaceae from Companion Animals, Beijing, China, 2012–2016
On This Page
Lei Lei1, Yang Wang1, Stefan Schwarz, Timothy R. Walsh, Yanran Ou, Yifan Wu, Mei Li, and Zhangqi Shen
Abstract
To investigate the prevalence of the recently emerging colistin resistance gene mcr-1 in Enterobacteriaceae among companion animals, we examined 566 isolates collected from cats and dogs in Beijing, China, during 2012–2016. Of these isolates, 49 (8.7%) were mcr-1–positive.
Multidrug-resistant and extensively drug-resistant gram-negative bacteria are a major threat to public health worldwide (1,2). The recent rapid dissemination of carbapenem-resistant Enterobacteriaceae has worsened this situation and further narrowed treatment options for infections caused by these bacteria (3). Colistin is a last-resort drug for treating carbapenem-resistant Enterobacteriaceae infections (4). In 2016, we identified the mobile colistin resistance gene mcr-1 (1). Soon after its description, mcr-1 was observed in Enterobacteriaceae from humans and food-producing animals in >30 countries on 5 continents (5).
A 2016 article reported that a 50-year-old man who worked in a pet store tested positive for mcr-1–harboring E. coli (6). Investigation identified 6 multidrug-resistant mcr-1–producing E. coli isolates in samples from 4 dogs and 2 cats in the pet store, indicating that the pathogens can be transmitted between humans and companion animals. So far, the prevalence of mcr-1-containing Enterobacteriaceae in companion animals is largely unknown. In our study, we focused on estimating the prevalence of mcr-1 in Enterobacteriaceae of companion animal origin in Beijing, China, during 2012–2016, and investigated the presence of the mcr-1 gene in pet foods purchased there.
In Beijing, the total number of registered dogs and cats is ≈1.2 million. We collected samples from both healthy and sick dogs and cats in Veterinary Teaching Hospital of China Agricultural University.
A total of 566 nonduplicate Enterobacteriaceae strains were isolated from 1,439 nasal and rectal swab samples collected from 1,254 dogs and 185 cats during 2012–2016. We also isolated 25 Enterobacteriaceae from 32 nasal swab samples from the pet owners. Because the food chain is among the main routes for humans and companion animals to acquire foodborne pathogens, we collected a small sample of pet foods (dog food, n = 30; cat food, n = 5) containing chicken as the main ingredient in Beijing during June–August 2016.
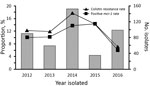
Figure. Proportion of colistin resistance and mcr-1 in Escherichia coli of companion animal origin, Beijing, China, 2012–2016.
The species of all Enterobacteriaceae were determined by 16S rDNA sequencing and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of specimens cultured on brain–heart infusion agar plates containing 2 μg/mL colistin. A total of 79/566 (14.0%) of the Enterobacteriaceae isolates from companion animals were resistant to colistin: 56 E. coli, 16 Klebsiella pneumoniae, 5 Enterobacter cloacae, 1 Enterobacter aerogenes, and 1 Shigella spp. PCR amplification of mcr-1 indicated that 8.7% (49/566) of Enterobacteriaceae and 62.0% (49/79) of colistin-resistant isolates harbored the mcr-1 gene, 47 E. coli and 2 K. pneumoniae. Only 1 E. coli isolate from a pet owner was colistin-resistant and mcr-1–positive. The proportions of mcr-1–containing E. coli per year ranged from 6.1% to 14.3% (Figure).
We examined the susceptibility of colistin-resistant E. coli to 8 other antimicrobial agents by agar dilution, according to the recommendations of Clinical and Laboratory Standards Institute (7). The mcr-1–carrying E. coli exhibited high resistance rates to ampicillin (97.9%), cefotaxime (91.5%), chloramphenicol (89.4%), and gentamicin (85.1%) (Technical Appendix[PDF - 354 KB - 2 pages]Table 1) but were susceptible to imipenem. The mcr-1–positive E. coli were more often resistant to amoxicillin/clavulanate, ampicillin, and chloramphenicol than were the mcr-1–negative E. coli (p<0.05) (Technical Appendix[PDF - 354 KB - 2 pages]Table 1).
All 57 colistin-resistant E. coli were subjected to XbaI pulsed-field gel electrophoresis (PFGE). The 55 colistin-resistant E. coli strains (2 nontypeable strains were excluded) were subdivided into 33 patterns and grouped into 31 clusters (A–Z, AB–AF) (online Technical Appendix Figure). The diversity and similarity of PFGE patterns of E. coli from different origins suggested that the dissemination of mcr-1 was possibly related to both clonal expansion and horizontal transmission.
Of note, the 1 E. coli colistin-resistant, mcr-1–positive isolate from a pet owner had the same PFGE pattern as 5 isolates from dogs and cats. Multilocus sequence typing linked these 6 strains to sequence type 101. These results suggest that E. coli strains can be exchanged between companion animals and humans.
The PCR and sequence analysis of the pet food samples showed that 7 of 35 samples were positive for mcr-1. Companies in China produced 5 of these foods; the other 2 were from Italy and Belgium (Technical Appendix[PDF - 354 KB - 2 pages]Table 2). These results suggest that pet foods may be a source from which intestinal bacteria of companion animals can acquire the mcr-1 gene.
Currently, colistin is not used to treat companion animals in China. The companion animals included in this study were from an urban area of Beijing, so they had minimal or no contact with food-producing animals in which colistin may have been used. Because we found mcr-1 in pet foods, we speculate that the pet food industry may be a source of mcr-1 among companion animals. Because of frequent and close contact between humans and companion animals, our study proposes that opportunities exist to transmit colistin-resistant Enterobacteriaceae to and from both groups. Thus, colistin-resistant Enterobacteriaceae from companion animals may represent a potential risk to human health. Further surveillance and control efforts are needed to reduce colistin-resistant and mcr-1–containing Enterobacteriaceae in companion animals and food-producing animals.
Ms. Lei is a PhD student in the College of Veterinary Medicine, China Agricultural University. Her main interest is the prevalence of antibiotic resistance in enteric bacteria.
Acknowledgment
This work was supported in part by National Natural Science Foundation of China (nos. 31672604, 31422055 and 81661138002). T.R. Walsh was also supported by MRC grant DETER-XDR-CHINA (MR/P007295/1).
References
- Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–8. DOIPubMed
- van der Bij AK, Pitout JD. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J Antimicrob Chemother. 2012;67:2090–100. DOIPubMed
- Temkin E, Adler A, Lerner A, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci. 2014;1323:22–42. DOIPubMed
- Michalopoulos AS, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas ME. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect. 2005;11:115–21. DOIPubMed
- Schwarz S, Johnson AP. Transferable resistance to colistin: a new but old threat. J Antimicrob Chemother. 2016;71:2066–70. DOIPubMed
- Zhang XF, Doi Y, Huang X, Li HY, Zhong LL, Zeng KJ, et al. Possible transmission of mcr-1-harboring Escherichia coli between companion animals and human. Emerg Infect Dis. 2016;22:1679–81. DOIPubMed
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; 3rd ed (VET01S). Wayne (PA): The Institute; 2005.
Figure
Technical Appendix
Cite This Article1These authors contributed equally to this article.






















.jpg)












No hay comentarios:
Publicar un comentario