
Volume 23, Number 4—April 2017
Dispatch
Detection and Molecular Characterization of Zoonotic Poxviruses Circulating in the Amazon Region of Colombia, 2014
On This Page
Jose A. Usme-Ciro, Andrea Paredes, Diana M. Walteros, Erica Natalia Tolosa-Pérez, Katherine Laiton-Donato, Maria del Carmen Pinzón, Brett W. Petersen, Nadia F. Gallardo-Romero, Yu Li, Kimberly Wilkins, Whitni Davidson, Jinxin Gao, Nishi Patel, Yoshinori Nakazawa, Mary G. Reynolds, P. S. Satheshkumar, Ginny L. Emerson, Andrés Páez-Martínez , and Andrés Páez Martínez.
, and Andrés Páez Martínez.
Abstract
During 2014, cutaneous lesions were reported in dairy cattle and farmworkers in the Amazon Region of western Colombia. Samples from 6 patients were analyzed by serologic and PCR testing, and results demonstrated the presence of vaccinia virus and pseudocowpox virus. These findings highlight the need for increased poxvirus surveillance in Colombia.
The Poxviridae family comprises large double-stranded DNA viruses that infect a wide range of invertebrate and vertebrate animals, including humans (1). Many poxviruses, particularly those belonging to the genus Orthopoxvirus (e.g., vaccinia virus [VACV]) and Parapoxvirus (e.g., pseudocowpox virus [PCPV]), are considered zoonotic viruses because their infections usually arise from human contact with infected domestic or sylvatic animal species (2).
Orthopoxviruses (OPXVs) gained considerable attention in the past because variola (smallpox) virus fatally infected millions of persons worldwide for centuries. However, in 1980, smallpox became the first infectious disease to be eradicated, resulting from vaccination campaigns involving several VACV strains (3). VACV has been detected in recurrent zoonotic outbreaks in Brazil, where it has been categorized into 2 well-defined genetic lineages (4). VACV has also emerged as a zoonotic virus in India (5). In 2011, VACV infections were found in cattle herds during active surveillance in Argentina (6). In Colombia, the most recently reported VACV outbreak in 1965–1966 caused disease in ≈8,570 cows and 150 humans and was associated with intensified smallpox eradication campaigns that extended until 1972 (3,7).
Parapoxviruses are emerging zoonotic pathogens that can cause infections in humans through direct contact with infected animals, especially domestic and wild ruminants (1). Among these viruses, bovine papular stomatitis virus, orf virus, and PCPV are known to cause cutaneous lesions (2). Although a parapoxvirus outbreak in imported goats was reported in Colombia in 1983 (8), only Brazil and the United States have formal evidence of endemic and zoonotic transmission of these viruses (9,10). We describe the clinical features, serologic and molecular diagnosis, and phylogenetic analysis of poxviruses circulating along the Amazon in western Colombia.

Figure 1. Location of 6 patients with poxvirus infections and photographs of lesions from 3 patients, Colombia, 2014. A) The municipalities of Valparaíso (residence of patients 1–5) and Solita (residence of patient 6)...
During an active outbreak investigation in February–July 2014, serum and exanthematous lesion samples were collected from 6 patients in the bordering municipalities of Valparaíso (patients 1–5) and Solita (patient 6) in the department of Caquetá (Figure 1; Table) in the Amazon Region of Colombia. Archived serum samples collected in December 2012–April 2013 from 11 patients with exanthematous lesions (from several farms) in the municipality of Valparaíso who consulted the local hospital were also analyzed (Table). In all patients, the incubation period ranged from 4–7 days, after which nodules on the hands or forearms appeared, along with fever, lymphadenopathy, and localized pain. Lesions increased in size and progressed from erythematous macules to papules, vesicles, and pustules that subsequently exhibited bacterial infection by the fourth week of symptom onset. By the fifth and sixth week, lesions progressed to scabs (Figure 1). Lesions appeared mainly in hands with preexisting cuts, abrasions, or other skin barrier defects. Direct contact with lesions from the udders of cattle during milking was reported by all but 1 patient. Patient 5 was a 24-year-old woman who reported no recent contact with infected animals, but her husband was a milker who had exanthematous lesions on his hands 2 weeks before she displayed symptoms.
All serum samples were tested by ELISA for OPXV IgG and IgM as described (11) (Technical Appendix[PDF - 258 KB - 4 pages]). An ELISA for detecting parapoxvirus-specific antibodies was not available. Fifteen serum samples were positive for OPXV IgG (Table); 9 were also positive for OPXV IgM, which can persist for up to 6 months after primary infection or vaccination (11). Reliable vaccination data was not available for these patients; however, on the basis of age, only patients 3, 8, and 11 could have been vaccinated. These 3 patients exhibited detectable OPXV IgG levels, and patients 3 and 11 were also positive for OPXV IgM. These results suggest that these patients had not been vaccinated and that their lesions were caused by recent OPXV exposure. Patients 4, 10, and 14 exhibited detectable OPXV IgG without detectable OPXV IgM. These results suggest the patients had previous OPXV infections, given that an active parapoxvirus infection was demonstrated in patient 4 and vaccination could be ruled out for patients 10 (a 20-year-old) and 14 (a 24-year-old) based on their age.
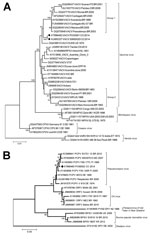
Figure 2. Phylogenetic characterization of orthopoxvirus gene A56R and parapoxvirus gene p37K of viruses obtained from patient lesion samples from an outbreak in Colombia, 2014. Trees were inferred by the neighbor-joining method. A)...
DNA was extracted from lesion and serum samples by using the PureLink viral RNA/DNA extraction kit (Invitrogen Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions and amplified by using PCR with generic and specific poxvirus primers as previously described (Technical Appendix[PDF - 258 KB - 4 pages]) (12). OPXV DNA was detected in lesion samples from patients 1, 2, and 5 (Table). For further genetic characterization, the A56R (hemagglutinin, 1,134 bp) gene from patients 2 and 5 were amplified by PCR, sequenced (Technical Appendix[PDF - 258 KB - 4 pages]), and compared with reference orthopoxvirus A56R sequences (Technical Appendix[PDF - 258 KB - 4 pages]Table 1). Our phylogenetic analysis confirmed VACV to be the etiologic agent of infection in these patients (Figure 2, panel A). The OPXV isolates detected in our samples were related to group 1 (Passatempo, Aracatuba, Muriae, and other strains from Brazil); however, bootstrap score and branch positioning suggest the strains from Brazil and Colombia diverged long ago or independently arose.
For parapoxvirus detection and characterization, we designed generic and degenerate primers to amplify a partial sequence of the p37K (envelope protein B2L, 489 bp) gene by PCR (Technical Appendix[PDF - 258 KB - 4 pages]). Parapoxvirus DNA was detected in lesion samples from patients 4 and 6 (Table). Nucleotide sequencing and phylogenetic analysis of the sample from patient 6 showed a close relationship with previously reported PCPV strains circulating in Brazil and worldwide (Technical Appendix[PDF - 258 KB - 4 pages]Table 2; Figure 2, panel B).
We found OPXV IgG and IgM in serum samples collected from patients with exanthematous lesions and from those who had such lesions in the past. Molecular methods allowed for the detection and characterization of VACV (3 patients) and PCPV (2 patients). Lesions caused by poxviruses affected the hands and forearms and disappeared within weeks without treatment. However, by limiting the patients’ daily activities, the infections had substantial negative impacts on the economies of dairy farmworkers and their local communities.
The disease burden of poxvirus infections in Colombia has not been estimated. However, anecdotal communications and data suggest it might be a serious and increasing health problem because farmworkers and healthcare personnel are not trained to recognize the disease and prevent subsequent transmission. The rapid spread of poxviruses could be facilitated by farmworkers performing daily milking activities at several farms and trading cattle. Also, factors such as human-to-human and fomite transmission, a growing susceptible population, and an unknown animal reservoir might increase the potential risk for infection at the community level. The serious consequences that poxvirus infections could have on immunocompromised persons (13), the increased risk for transmission related to the anatomic site of the lesions (14), the potential for long-lasting sequelae, and the possibility of the virus evolving into a more virulent strain (15) could pose a great threat to individual persons, populations, and public health systems in the near future.
Studies focused on determining the prevalence of poxvirus infections, risk factors for disease, and the geographic distribution of poxvirus strains are needed to understand the disease burden and guide effective prevention and control measures and educational outreach. Analysis and characterization of VACV and PCPV complete genomes could provide clues to explain their emergence and recent evolutionary histories, and research aimed at identifying the domestic animal hosts and wildlife reservoirs of poxviruses could further our understanding of their natural transmission cycles.
Dr. Usme-Ciro was the founder of the Unit of Sequencing and Genomics at the National Institute of Health in Colombia. His research is focused on molecular virology, specifically on molecular epidemiology, evolution and emergence of viruses of public health relevance in Colombia, and the study of viral determinants of disease severity.
References
- Damon IK. Poxviruses. In: Knipe DM, Howley PM, editors. Fields virology. New York: Lippincott Williams & Wilkins; 2007.
- Lewis-Jones S. Zoonotic poxvirus infections in humans. Curr Opin Infect Dis. 2004;17:81–9. DOIPubMed
- Fenner F, Henderson DA, Arita I, Jezek A, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organization Press; 1988.
- Oliveira DB, Assis FL, Ferreira PC, Bonjardim CA, de Souza Trindade G, Kroon EG, et al. Group 1 Vaccinia virus zoonotic outbreak in Maranhao State, Brazil. Am J Trop Med Hyg. 2013;89:1142–5. DOIPubMed
- Bhanuprakash V, Venkatesan G, Balamurugan V, Hosamani M, Yogisharadhya R, Gandhale P, et al. Zoonotic infections of buffalopox in India.Zoonoses Public Health. 2010;57:e149–55. DOIPubMed
- Franco-Luiz AP, Fagundes-Pereira A, Costa GB, Alves PA, Oliveira DB, Bonjardim CA, et al. Spread of vaccinia virus to cattle herds, Argentina, 2011.Emerg Infect Dis. 2014;20:1576–8. DOIPubMed
- Gómez Pando V, Hernán López J, Restrepo A, Forero P. [Study of an outbreak of vaccinia in dairy cattle of their milkers]. Bol Oficina Sanit Panam. 1967;63:111–21.PubMed
- Rodríguez G, Moreno O, Mogollón JD, Latorre S, Cortés E. ORF: un brote en cabras importadas. Biomedica. 1983;3:49–57. DOI
- Schatzmayr HG, Lemos ER, Mazur C, Schubach A, Majerowicz S, Rozental T, et al. Detection of poxvirus in cattle associated with human cases in the State of Rio de Janeiro: preliminary report. Mem Inst Oswaldo Cruz. 2000;95:625–7. DOIPubMed
- Delhon G, Tulman ER, Afonso CL, Lu Z, de la Concha-Bermejillo A, Lehmkuhl HD, et al. Genomes of the parapoxviruses ORF virus and bovine papular stomatitis virus. J Virol. 2004;78:168–77. DOIPubMed
- Karem KL, Reynolds M, Braden Z, Lou G, Bernard N, Patton J, et al. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–72.PubMed
- Li Y, Meyer H, Zhao H, Damon IK. GC content-based pan-pox universal PCR assays for poxvirus detection. J Clin Microbiol. 2010;48:268–76. DOIPubMed
- Redfield RR, Wright DC, James WD, Jones TS, Brown C, Burke DS. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–6. DOIPubMed
- Lewis FMT, Chernak E, Goldman E, Li Y, Karem K, Damon IK, et al. Ocular vaccinia infection in laboratory worker, Philadelphia, 2004. Emerg Infect Dis. 2006;12:134–7. DOIPubMed
- Shchelkunov SN. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013;9:e1003756. DOIPubMed






















.png)












No hay comentarios:
Publicar un comentario