Shattering News: How Chromothripsis Cured a Rare Disease
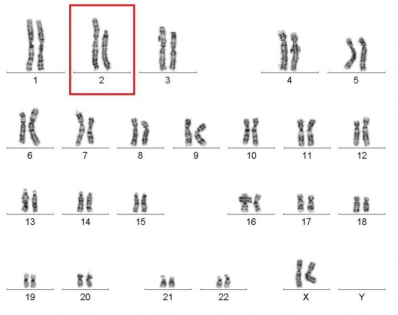
Caption: Karyotype of a woman spontaneously cured of WHIM syndrome. These chromosome pairings, which are from her white blood cells, show a normal chromosome 2 on the left, and a truncated chromosome 2 on the right.
Source: National Institute of Allergy and Infectious Diseases , NIH
Source: National Institute of Allergy and Infectious Diseases , NIH
The world of biomedical research is filled with surprises. Here’s a remarkable one published recently in the journal Cell [1]. A child born in the 1950s with a rare genetic immunodeficiency syndrome amazingly cured herself years later when part of one of her chromosomes spontaneously shattered into 18 pieces during replication of a blood stem cell. The damaged chromosome randomly reassembled, sort of like piecing together a broken vase, but it was still missing a shard of 164 genes—including the very gene that caused her condition.
Researchers say the chromosomal shattering probably took place in a cell in the bone marrow. The stem cell, now without the disease-causing gene, repopulated her immune system with healthy bone marrow-derived immune cells, resulting in cure of the syndrome.
How is this possible? The research story begins a couple of years ago when the woman, now 59, contacted researchers at NIH’s National Institute of Allergy and Infectious Diseases (NIAID), who study a rare immunodeficiency known as WHIM syndrome. The woman reported that she and two of her daughters had the autosomal dominant disorder.
It isn’t every day that researchers get a call out of the blue from someone with WHIM syndrome: fewer than 60 cases have been diagnosed worldwide over the past 50 years. The interaction took an even more interesting turn when the researchers learned that the mother was the first person ever diagnosed with the condition as a child in 1964 [2, 3]. She’d been through the wringer with the syndrome, battling its puzzling manifestations of serious recurrent infections and skin warts caused by an unexplained susceptibility to the human papillomavirus.
When asked how she was doing now, the mother responded that the syndrome had vanished during her 30s. Philip Murphy, a NIAID immunologist, said he was floored by her comment—it just didn’t seem medically possible.
Everyone is born with two copies of a gene called CXCR4. The gene encodes a cell-surface receptor that mediates immune signaling. But people born with WHIM syndrome inherit one normal and one mutant copy of the gene [4]. The garbled gene yields an abnormal receptor that signals excessively on the surface of white blood cells. It’s like a car with the accelerator stuck. The excess signaling blocks the ability of the young white blood cells in the bone marrow (monocytes and neutrophils) to mature, preventing an adequate supply from entering the blood stream to help fight off infections.
After some initial tests on the family, Murphy and his colleagues noted that the mother’s white cells were no longer deficient in numbers – which is not expected in WHIM syndrome. They reasoned that the only way the mother could have been cured was if the mutant copy of her CXCR4gene was lost somehow. So David McDermott and Joy Liu from Murphy’s lab examined the woman’s white blood cells, and sure enough, the abnormal copy was gone. But when they examined cells from other tissues of her body, the mutant gene was still present. That’s how two of her three daughters inherited the condition.
With further DNA testing, the NIAID team noticed a truncated and massively rearranged chromosome 2 (shown in the image above) in the mother’s white cells (but not in cells from her skin). They developed a solid but rather astonishing explanation. Years ago, they deduced that in a single blood-forming stem cell in the woman’s marrow, part of chromosome 2 must have undergone a major breakage and reassembly. The name of this phenomenon is chromothripsis, which in Greek means “a chromosome shattering into pieces.”
Chromothripsis was originally identified in cancer cells, and is now known to occur in about 2 percent of cancers. Since then, it’s also been identified in a patient with severe congenital cognitive syndrome. What scientists had not imagined is that such a cataclysmic event could actually cure a disease. Murphy hypothesized that the loss of the mutant CXCR4 gene gave a stem cell an advantage to grow, divide, and eventually replace all of the woman’s stem cells carrying the WHIM harmful mutation.
There currently are no approved treatments for WHIM syndrome. But knowing about this unique case suggests that manipulation of bone marrow stem cells to inactivate the mutant gene, perhaps using the CRISPR-Cas system (See “Copy-Editing the Genome“), might provide a new strategy in the future. Researchers also suggest that the findings might also help improve bone marrow transplantation, which relies on the ability of donor stem cells to repopulate in a transplant recipient.
References:
[1] Chromothriptic cure of WHIM syndrome. McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N, Dai Z, Marquesen MM, Stregevsky E, Kwatemaa N, Theobald N, Long Priel DA, Pittaluga S, Raffeld MA, Calvo KR, Maric I, Desmond R, Holmes KL, Kuhns DB, Balabanian K, Bachelerie F, Porcella SF, Malech HL, Murphy PM. Cell. 2015 Feb 12;160(4):686-99.
[2] “Myelokathexis’’– A New Form of Chronic Granulocytopenia. Report of a Case. Zuelzer WW. N Engl J Med. 1964 Apr 2;270:699-704.
[3] Chronic Idiopathic Granulocytopenia. Krill CE Jr, Smith HD, Mauer AM. N Engl J Med. 1964 May 7;270:973-9.
[4] Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Nat Genet. 2003 May;34(1):70-4.
Links:
Primary Immunodeficiency (Eunice Kennedy Shriver National Institute of Child Health and Development/NIH)
Office of Rare Diseases (National Center for Advancing Translational Sciences/NIH)
Philip M. Murphy, National Institute of Allergy and Infectious Diseases, Bethesda
NIH Support: National Institute of Allergy and Infectious Diseases; National Cancer Institute; National Heart, Lung, and Blood Institute





















.png)












No hay comentarios:
Publicar un comentario