
Trial Should Change Care for AIDS-Related Kaposi Sarcoma in Sub-Saharan Africa
, by NCI Staff
Results from a large clinical trial are expected to establish a new standard treatment for people with AIDS in sub-Saharan Africa who have advanced forms of Kaposi sarcoma. In the trial, treatment with the chemotherapy drug paclitaxel greatly improved the outcomes of those with advanced AIDS-related Kaposi sarcoma compared with the chemotherapy drugs typically used in these countries.
From a public health perspective, the results are incredibly important, said the trial’s lead investigator, Susan Krown, M.D., of the NCI-funded AIDS Malignancy Consortium (AMC). Kaposi sarcoma, which most often forms on the skin but in more severe cases can involve internal organs, is one of the most common cancers in many of the countries in sub-Saharan Africa, and it’s most often diagnosed in those with AIDS.
Dr. Krown noted that the limited health care infrastructures in many countries in sub-Saharan Africa make implementing changes in care a challenge. “But I think practice will move more toward the wider use of paclitaxel to treat AIDS-related Kaposi sarcoma based on these findings,” she said.
The results, published March 5 in The Lancet, were unexpected, said Robert Yarchoan, M.D., director of NCI’s Office of HIV and AIDS Malignancy. When the trial was launched more than 7 years ago, there was little data on how the different treatments being used in the trial compared against one another.
“But what they showed was that one, paclitaxel, was much better than the others,” Dr. Yarchoan said.
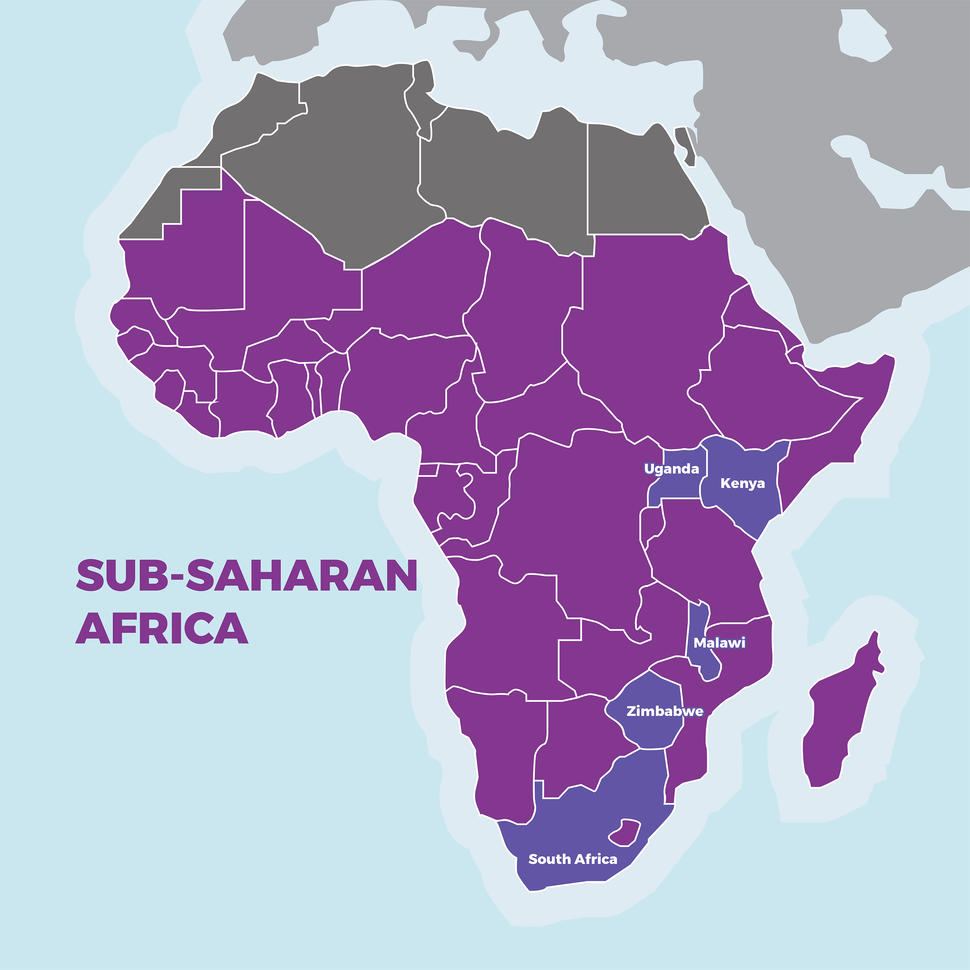
NCI and the National Institute of Allergy and Infectious Diseases (NIAID) funded the trial. It was conducted in five sub-Saharan African countries (Uganda, Zimbabwe, Malawi, Kenya, and South Africa) and Brazil, which also has high rates of AIDS-related Kaposi sarcoma.
The findings come at a good time, with the improved availability and affordability of paclitaxel and other chemotherapy drugs in sub-Saharan Africa, said Satish Gopal, M.D., M.P.H., director of NCI’s Center for Global Health, who was not directly involved in the trial. Dr. Gopal previously lived and worked in Malawi as part of a longstanding research collaboration between the University of North Carolina and Malawi Ministry of Health, where for a time he was the only certified medical oncologist in a country with a population of more than 18 million.
These findings also provide much-needed scientific evidence to drive clinical decision-making, Dr. Gopal continued. Without such evidence, he explained, cancer treatment in sub-Saharan Africa is too often dictated by drug availability and extrapolation of data from studies done in entirely different patient populations in high-income countries.
With these new results, he said, “clinicians and governments in the region can feel comfortable making informed treatment decisions, and developing practice guidelines and policies, that are grounded in a rigorously conducted study done in sub-Saharan Africa.”
The Burden of AIDS-Related Kaposi Sarcoma
Tremendous progress has been made against HIV, both in preventing the infection and in treating AIDS, the disease it causes. This holds true for highly developed countries like the United States as well as in low- and middle-income countries around the globe.
Nevertheless, in sub-Saharan Africa, AIDS-related malignancies like Kaposi sarcoma remain a significant health burden. In Uganda, for example, Kaposi sarcoma is the most common cancer in men and the second most common in women.
This is due to widespread presence in this region of Kaposi sarcoma-associated herpesvirus, which causes Kaposi sarcoma, and HIV, which makes people particularly susceptible to developing this cancer, noted Dr. Gopal.
While Kaposi sarcoma is very rare in the United States, in many sub-Saharan African countries “it’s truly a public health problem that’s on the same order of magnitude of colorectal cancer or lung cancer in the United States,” he said.
Most countries in this region have limited and under-resourced health care systems that often cannot provide treatments that are readily available in the United States and other highly developed countries. For instance, in Malawi, access to radiation therapy—a mainstay treatment for many cancers—is currently nonexistent.
In people with AIDS-related Kaposi sarcoma, HIV-directed treatment—known as antiretroviral therapy (ART)—can be effective as a stand-alone treatment in mild cases. But in more advanced cases, ART alone is insufficient and is often combined with chemotherapy, Dr. Yarchoan said. In sub-Saharan Africa, most patients are diagnosed with advanced disease.
In the United States, either paclitaxel or liposomal doxorubicin is usually combined with ART to treat advanced AIDS-related Kaposi sarcoma. But, in large part because of their expense, neither drug has been available in sub-Saharan Africa.
And despite the tremendous burden of AIDS-related Kaposi sarcoma there, little data were available on the efficacy of the chemotherapy regimens most commonly used to treat advanced AIDS-related Kaposi sarcoma, either etoposide alone or bleomycin used in combination with vincristine.
According to Dr. Yarchoan, that knowledge gap created an opportunity that NIAID and NCI—through their AIDS Clinical Trials Group and AMC, respectively—felt they could address.
A Much-Needed Trial
From a broader scientific and clinical standpoint, Dr. Yarchoan stressed, there was a critical need for this clinical trial. No large trials of treatment for Kaposi sarcoma had been conducted in their entirety since ART was widely adopted more than 20 years ago. And it was the first large randomized trial comparing chemotherapy regimens for the treatment of AIDS-related Kaposi sarcoma to be completed in more than a decade.
The initial planning for the trial actually began in the mid-2000s, Dr. Krown said, and it had been set to launch in 2011.
But a shortage of liposomal doxorubicin at the time, which was supposed to be the drug that was compared against the two commonly used chemotherapy regimens, disrupted that plan. When it was clear that the shortage would not be resolved quickly, trial investigators shifted gears and took the steps needed to use paclitaxel instead. With that change, they were able to launch the trial in 2013.
But conducting clinical trials in sub-Saharan Africa has its challenges, Dr. Krown explained, in part because medical staff there have limited experience with research studies. “A lot of time was invested in training staff at the [trial] sites,” she said. “There was a lot of training of clinicians, nurses, and pharmacists, and there was a lot of infrastructure building.”
More than 330 patients enrolled in the trial, with each randomly assigned to receive paclitaxel, etoposide, or bleomycin and vincristine, in addition to ART.
Participants treated with paclitaxel lived substantially longer without their disease getting worse (progression-free survival) than those who received the other two treatments, particularly those treated with etoposide. At 48 weeks after beginning treatment, progression-free survival was 50% in the paclitaxel group compared with 20% in the etoposide group.
In addition, patients who received paclitaxel were far more likely to experience reductions in the size of their tumors (tumor response) than patients who received the other two chemotherapy regimens. The length of time those tumor responses lasted was also greater.
Overall, patients treated with paclitaxel tolerated the drug well, Dr. Krown and her colleagues reported. For logistical reasons, paclitaxel was given less frequently than it is in the United States—once every 3 weeks rather than once every 2 weeks.
This lower “treatment intensity,” however, didn’t translate into less efficacy, they reported. The tumor response rates in this trial were the same as those seen in a large trial conducted in the United States that used a once-every-2-weeks treatment schedule. It did, however, lead to much lower incidence of neutropenia—a drop in levels of white blood cells known as neutrophils, which increases the risk of infections—than was seen with paclitaxel in the US trial.
The finding of a clear difference among these regimens “really highlights the fact that we can learn things from trials in less-developed countries that can apply in the United States and throughout the world,” Dr. Yarchoan said.
And the liposomal doxorubicin shortage that slowed the launch of the trial appears to have had a silver lining, Dr. Krown said, since paclitaxel is now becoming more available in sub-Saharan Africa. “At the time, it didn’t seem to be lucky, but things worked out well.”
From a Trial to Everyday Care
It’s one thing to complete a clinical trial and publish the results. It’s another altogether to see those results translated into everyday care. But a confluence of activities and initiatives should help in this regard, Dr. Yarchoan said.
In particular, several years ago the American Cancer Society (ACS) and Clinton Health Access Initiative launched an effort to improve the affordability and accessibility of 16 common cancer drugs, including paclitaxel, in sub-Saharan Africa. Although still in its early stages, the initiative is off to a good start, with ACS reporting that several countries are achieving significant cost savings on the drugs.
Dr. Krown confirmed that, although in some sub-Saharan countries the cost of paclitaxel can still be prohibitive, “it’s now much more widely available.”
While paclitaxel may now be more accessible, there are other barriers for patients in this region, wrote Esther Freeman, M.D., and Devon McMahon of Harvard Medical School, and Miriam Laker-Oketta, M.D., of Makerere University in Uganda, in an accompanying editorial in The Lancet. For example, other expenses related to chemotherapy and its administration, including travel to treatment sites, were covered as part of the trial, and participants received regular home visits from medical staff.
To ensure that these findings are implemented in everyday patient care, they wrote, further efforts will be needed “to devise strategies that tackle cost, transportation, and [treatment] adherence barriers.”
Nevertheless, Dr. Gopal said, completing the trial is a major achievement, both for current patient care and to help inform future research.
“I think it’s a really important demonstration that … paclitaxel really should be the regional standard of care for treating this cancer,” he said. It also shows that high-quality cancer treatment trials can be done in Africa, he added. “Those are important milestones that have now generated the type of evidence that will directly inform policies, programs, and future studies in that part of the world.”





















.png)












No hay comentarios:
Publicar un comentario