
Volume 26, Number 2—February 2020
Dispatch
Influence of Rainfall on Leptospira Infection and Disease in a Tropical Urban Setting, Brazil
On This Page
Article Metrics
Kathryn P. Hacker1, Gielson A. Sacramento1, Jaqueline S. Cruz, Daiana de Oliveira, Nivison Nery, Janet C. Lindow, Mayara Carvalho, Jose Hagan, Peter J. Diggle, Mike Begon, Mitermayer G. Reis, Elsio A. Wunder, Albert I. Ko1, and Federico Costa1
Abstract
The incidence of hospitalized leptospirosis patients was positively associated with increased precipitation in Salvador, Brazil. However, Leptospira infection risk among a cohort of city residents was inversely associated with rainfall. These findings indicate that, although heavy rainfall may increase severe illness, Leptospira exposures can occur year-round.
Leptospirosis, a leading zoonotic cause of illness and death (1), has emerged as a major health problem due to the global expansion of urban slum communities (2–4). The disease is associated with severe manifestations such as Weil’s disease and pulmonary hemorrhage syndrome (5), for which case-fatality rates are 10%–50% or even higher (6). Transmission to slum residents occurs in the peridomiciliary environment, in which exposures to sewers, floodwater, and contaminated soil are risk factors (3,7,8). Extreme weather events may precipitate outbreaks (3–6), as recently experienced during the aftermath of Hurricane Maria in Puerto Rico (9). Similarly, seasonal periods of heavy rainfall and flooding are a contributing factor to the risk for urban leptospirosis (4,10).
In urban slum settings, contact with rats and Leptospira-contaminated water and soil occur year-round (3). Prior studies have shown, consistently, positive associations between heavy rainfall and hospitalized leptospirosis case-patients (4,10). However, this relationship may be affected by differences in case definitions used by diverse surveillance systems. In the few prospective cohort studies available, estimates of severe disease accounted for only a small proportion of the total disease burden (6). Thus, little is known about the role of rainfall in overall infection rates. To characterize the seasonal pattern of leptospirosis and Leptospira infection in a tropical urban setting and evaluate the influence of meteorological factors on seasonal risk, we conducted a prospective investigation of Leptospira infection rates among slum residents while actively surveying for hospitalized leptospirosis case-patients within Salvador, Brazil, during seasonal periods of high and low rainfall.
During February 2013–April 2015, we identified patients >5 years old with suspected leptospirosis at the state infectious disease hospital in Salvador, Brazil (4,5), and those reported in the public health surveillance database by other hospitals in Salvador. We estimated the probable date of infection as 15 days before the hospital admission date. We evaluated suspected leptospirosis cases according to the WHO case definition standard (4,6,11) using the microscopic agglutination test (MAT), lipL32 real-time PCR assay (11), IgM-ELISA (6), or a combination. We defined laboratory-confirmed cases of leptospirosis as those with >4-fold rise in MAT titers in paired serum samples, MAT titers >1:800 in a single sample, or positive PCR (Appendix Tables 1, 2).
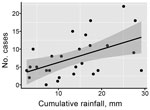
A linear regression model identified that cumulative monthly rainfall (Figure 1, panel A) was significantly associated with the monthly number of hospitalized cases (r2 = 0.22, p<0.007) (Figure 2). The highest hospitalized disease incidence occurred during the first period (February–September 2013; 3.29 cases/100,000 population; 95% CI 2.67–4.01 cases/100,000 population) and decreased across the next periods (Table 1; Figure 1, panels B, C).
Concurrently, we conducted a prospective cohort study assessing serologic evidence of Leptospira infection among urban slum residents of Pau da Lima, northwestern Salvador. We enrolled 2,421 of 3,716 eligible residents, >5 years of age and with written informed consent, of whom 821 participated in all serologic surveys performed twice annually during August–September (dry season) and February–March (rainy season) during 2013–2015 (Figure 1, panel A). Using panels with the 2 most common Leptospira species in Salvador (4), L. interrogans serogroup Icterohaemorrhagiae serovar Copenhageni (strain Fiocruz L130) and L. kirsheri serogroup Cynopteri serovar Cynopteri (strain 3522C), we defined serologic evidence of Leptospira infection by a MAT titer increase from negative to >1:50 (seroconversion) or >4-fold increase between sequential, paired samples. During the study period, 29% of the infected participants reported fever.
To assess the association between rainfall and laboratory-confirmed Leptospira infection, we calculated the cumulative amount of rainfall that each study participant experienced between sequential samples. We used a generalized estimating equation and incorporated explanatory variables for gender, age, time period, and cumulative rainfall that each participant experienced. In contrast to the hospitalized cases, we found Leptospira infection risk in the urban area had an inverse association with cumulative rainfall (0.986 cm, 95% CI 0.977–0.995 per cm) (Table 2; Figure 1, panel D). We additionally assessed various rainfall metrics, as well as the number of severe rainfall events each participant experienced above the mean rainfall, and the resulting patterns remained consistent. Increasing age and male sex were associated with higher infection risk.
Leptospirosis is traditionally associated with heavy rainfall and flooding events in Brazil (5,9) and worldwide (7,10). Our findings support the association between extreme weather events and clinical leptospirosis. During the study period, the risk of acquiring leptospirosis that required hospitalization was significantly higher in periods with elevated rainfall. However, this finding is in contrast to Leptospira infection in nonhospitalized persons.
Our findings indicate that Leptospira infections occur year-round in this urban tropical setting and the cumulative incidence of Leptospira infection is high (2%–9% per period). This finding differs from patterns that we and others have identified for leptospirosis requiring hospitalization (2,4,9,12). Although this study does not specifically assess subclinical symptomatic infection, it provides further evidence that the impact of leptospirosis is underestimated, and physicians should be aware that leptospirosis infection may manifest clinically year-round.
The patterns of Leptospira exposure incidence and infection severe enough to require hospitalization, when taken together, suggest that rainfall may promote exposures of greater inocula, which in turn may increase the risk of developing severe clinical outcomes, such as severe pulmonary hemorrhage syndrome and Weil’s disease. For example, heavy rainfall may diffuse Leptospira from the soil, resulting in higher concentrations of bacteria in the media to which humans are exposed (sewer water) and so to a higher inoculum dose, thus increasing hospitalized disease incidence and perhaps decreasing the environmental exposure risk in and around households (mud and exposed soil) and decreasing infection risk. However, additional studies are needed to assess the specific contribution of inoculum dose to disease severity.
The 2-year study period was atypical because rainfall was lower than expected during the rainy seasons (Figure 1, panel A; Appendix Figure 1). Of note, we observed a significant inverse association between cumulative rainfall and the risk for infection during biannual sampling periods. Thus, these trends may not apply to periods with higher amounts of rainfall or extreme climatic events, such as El Niño. This study was also limited because we used seroconversion to identify infection and therefore could not determine the precise timing of exposure events; furthermore, we conducted serologic surveys only in a single urban slum community. However, most hospitalized cases occur in similar communities (4), and therefore Pau da Lima is likely to be representative. Last, although the surveillance hospitals were able to capture a variety of febrile illnesses, they did not capture mild febrile illness, which may account for a missing proportion of leptospirosis cases.
Our findings demonstrate that, despite the association of leptospirosis hospitalization with rainfall, Leptospira exposure continues year-round. Although we did not evaluate mild subclinical or clinical infections, it is possible that participants experience symptomatic illness that may be unrecognized or misdiagnosed as dengue or other febrile disease (12,13). Clinicians should be aware that leptospirosis may manifest clinically outside of normal seasonal periods of heavy rainfall. In addition, the differences observed during the time periods independent from rainfall indicate that other unexplained factors may influence the temporal risk for Leptospira infection. Identifying these factors will help enhance intervention strategies in urban slum environments.
While developing this work, Dr. Hacker was a PhD candidate in the Department of Epidemiology of Microbial Disease at Yale University. She is a postdoctoral fellow at the University of Pennsylvania focusing on risk mapping of zoonotic diseases in complex urban settings in the Department of Biostatistics, Epidemiology, and Informatics. Mr. Sacramento is pursuing a PhD in biotechnology in health and investigative medicine at Fiocruz Brazil. His research interests include the epidemiology of zoonotic disease, transmission dynamics, and environmental dynamics affecting the transmission of disease in urban communities.
Acknowledgments
We thank the Community Health Council of Pau da Lima and the members of the Pau da Lima community in Salvador, Brazil. We also thank team members from the Urban Health Council of Pau da Lima and Oswaldo Cruz Foundation.
The study was supported by grants from the Fogarty International Center (R25 TW009338, R01 TW009504) and National Institute of Allergy and Infectious Diseases (F31 AI114245, R01 AI121207) from the National Institutes of Health; the UK Medical Research Council (MR/P0240841); the Wellcome Trust (102330/Z/13/Z); and the Fulbright Foundation.
References
- Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9:
e0003898 . - Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al.; Peru-United States Leptospirosis Consortium. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–71.
- Hagan JE, Moraga P, Costa F, Capian N, Ribeiro GS, Wunder EA Jr, et al. Spatiotemporal determinants of urban leptospirosis transmission: four-year prospective cohort study of slum residents in Brazil. PLoS Negl Trop Dis. 2016;10:
e0004275 . - Ko AI, Galvão Reis M, Ribeiro Dourado CM, Johnson WD Jr, Riley LW; Salvador Leptospirosis Study Group. Urban epidemic of severe leptospirosis in Brazil. Lancet. 1999;354:820–5.
- Gouveia EL, Metcalfe J, de Carvalho AL, Aires TS, Villasboas-Bisneto JC, Queirroz A, et al. Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerg Infect Dis. 2008;14:505–8.
- McBride AJA, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–86.
- Felzemburgh RD, Ribeiro GS, Costa F, Reis RB, Hagan JE, Melendez AX, et al. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the Leptospira agent. PLoS Negl Trop Dis. 2014;8:
e2927 . - Reis RB, Ribeiro GS, Felzemburgh RDM, Santana FS, Mohr S, Melendez AXTO, et al. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl Trop Dis. 2008;2:
e228 . - Centers for Disease Control and Prevention. 2017 hurricane key messages. Atlanta: Centers for Disease Control and Prevention; 2017 [cited 2019 Dec 17].
- Maskey M, Shastri JS, Saraswathi K, Surpam R, Vaidya N. Leptospirosis in Mumbai: post-deluge outbreak 2005. Indian J Med Microbiol. 2006;24:337–8.
- Riediger IN, Stoddard RA, Ribeiro GS, Nakatani SM, Moreira SDR, Skraba I, et al. Rapid, actionable diagnosis of urban epidemic leptospirosis using a pathogenic Leptospira lipL32-based real-time PCR assay. PLoS Negl Trop Dis. 2017;11:
e0005940 . - Lau CL, Smythe LD, Craig SB, Weinstein P. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Trans R Soc Trop Med Hyg. 2010;104:631–8.
- Flannery B, Pereira MM, Velloso L de F, Carvalho C de C, De Codes LG, Orrico G de S, et al. Referral pattern of leptospirosis cases during a large urban epidemic of dengue. Am J Trop Med Hyg. 2001;65:657–63.
Figures
Tables
Cite This ArticleOriginal Publication Date: 1/7/2020
1These authors contributed equally to this article.



































No hay comentarios:
Publicar un comentario