
Volume 26, Number 2—February 2020
Research
Cost-effectiveness of Screening Program for Chronic Q Fever, the Netherlands
On This Page
Article Metrics
Pieter T. de Boer , Marit M.A. de Lange, Cornelia C.H. Wielders, Frederika Dijkstra, Sonja E. van Roeden, Chantal P. Bleeker-Rovers, Jan Jelrik Oosterheert, Peter M. Schneeberger, and Wim van der Hoek
, Marit M.A. de Lange, Cornelia C.H. Wielders, Frederika Dijkstra, Sonja E. van Roeden, Chantal P. Bleeker-Rovers, Jan Jelrik Oosterheert, Peter M. Schneeberger, and Wim van der Hoek
Abstract
In the aftermath of a large Q fever (QF) epidemic in the Netherlands during 2007–2010, new chronic QF (CQF) patients continue to be detected. We developed a health-economic decision model to evaluate the cost-effectiveness of a 1-time screening program for CQF 7 years after the epidemic. The model was parameterized with spatial data on QF notifications for the Netherlands, prevalence data from targeted screening studies, and clinical data from the national QF database. The cost-effectiveness of screening varied substantially among subpopulations and geographic areas. Screening that focused on cardiovascular risk patients in areas with high QF incidence during the epidemic ranged from cost-saving to €31,373 per quality-adjusted life year gained, depending on the method to estimate the prevalence of CQF. The cost per quality-adjusted life year of mass screening of all older adults was €70,000 in the most optimistic scenario.
Chronic Q fever (CQF) is a potentially lethal condition that develops in 2% of Q fever (QF) patients (1). QF is caused by infection with Coxiella burnetii, a gram-negative bacterium that has its main reservoir in livestock and can infect humans by airborne transmission. CQF can become apparent months to years after infection and usually manifests as endocarditis or vascular infection (2). Risk factors for CQF include heart valve disorders, aortic aneurysms, vascular prostheses, older age, and a compromised immune system (3–5). Prognosis is poor despite long-term antimicrobial drug treatment; 28% of patients need surgery, and 15% die from CQF-related complications (6).
During 2007–2010, the Netherlands faced the world’s largest QF epidemic ever documented. More than 4,000 patients with acute QF were notified. However, QF often occurs asymptomatically (1), and the total number of infections has been estimated at 50,000 (7). Through May 2016, a substantial number of CQF infections occurred, and at least 74 patients died (8). Because early detection of CQF might result in a better prognosis, local hospitals initiated multiple targeted screening studies for clinical risk groups living in areas affected by the epidemic. These studies revealed that 7%–20% of screened patients had serologic evidence of C. burnetii infection, of whom 5%–31% had CQF (9–11).
In 2017, new diagnoses of CQF continued to appear in the Netherlands, often with severe complications, and led to a call from multiple concerned parties, including politicians, the QF patient association, and medical doctors for a national CQF screening program. One aspect considered for such a screening program is whether its costs are economically balanced with the expenditure (12,13). To answer this question, we assessed the cost-effectiveness of a screening program for CQF in the Netherlands.
Overview
We developed a health-economic decision model to compare estimated costs and effects of a 1-time screening program for CQF with no such screening program (Figure 1). The screening was assumed to occur in 2017, seven years after the epidemic. We estimated comparative outcomes of the model in terms of clinical events, quality-adjusted life years (QALYs), and costs from a societal perspective. We used a lifetime time horizon. Costs were annually discounted at 4% and QALYs at 1.5% (14).
Screening Population
The analysis focused on adults >18 years years of age. Because the prevalence of CQF is not uniformly distributed in the population (most QF patients resided in the south of the Netherlands; patients can have risk factors for CQF), we considered different subgroups for screening. We used the Netherlands population data from 2017 (15). First, we stratified the population on the basis of residence area between high, middle, and low QF incidence areas. For this stratification, we used spatial data on QF notifications and farms with QF outbreaks during the epidemic period (2007–2010). Next, we further divided these subgroups on the basis of a risk factor for CQF between persons with a cardiovascular risk factor, an immunocompromised status, or an unknown risk status. The last group was labeled as unknown because the prevalences of heart valve disorders and aortic aneurysms are underreported. Because these cardiovascular prevalences increase with age, the unknown subgroup was split between persons <60 years and >60 years of age. Thus, we considered 12 (3 × 4) subgroups (Table 1). We obtained prevalences of diagnosed and undiagnosed risk factors from the literature (16–21) (Appendix Table 1).
Model
We used a decision-tree model that consisted of 2 parts: a screening part and a clinical part (Appendix Figure 1). CQF is usually characterized by persistent high IgG against C. burnetii phase I, often in the presence of high IgG against phase II (2,3). In the current clinical setting in the Netherlands, patients suspected of having CQF are tested with immunofluorescence assay (IFA) for IgG against phase I. However, IFA is a nonautomated and subjective test, and its use might not be feasible for a large-scale screening program (22). Therefore, we proposed an initial screening round with the ELISA for IgG against phase II, and positive samples were tested with IFA for IgG against phase I. In the sensitivity analysis, we explored a scenario with direct testing with IFA for IgG against phase I.
In the clinical part, patients were first classified among proven, probable, or possible CQF, according to the guideline of the Dutch Q Fever Consensus Group (23). This classification ranks the probability of having CQF based on PCR, serology, clinical parameters, imaging techniques, and pathologic findings (Appendix Table 2). Next, patients were divided by focus of infection and whether CQF led to an early complication (before diagnosis or within 12 weeks after diagnosis). Complications considered were heart failure, symptomatic aneurysm, arterial embolic complication, and other complications. After diagnosis, antimicrobial treatment can be initiated, possibly combined with a surgical procedure. Then, patients may have a late complication (>12 weeks after diagnosis) and can die of CQF.
CQF Prevalence
The prevalence of CQF 7 years after the QF epidemic is uncertain because the average duration between infection and development of CQF is unknown. Therefore, we considered 2 scenarios, a low CQF prevalence scenario and a high CQF prevalence scenario. For both scenarios, we estimated the prevalence of CQF in 3 consecutive steps: 1) define the risk for C. burnetii infection per QF incidence area, 2) multiply by the risk for CQF given infection per risk group, and 3) adjust the CQF prevalence from directly after the epidemic to the year of screening 7 years later. This final step accounts for a decrease of CQF prevalence over time, for instance, because of death or earlier diagnosis.
We selected parameter values for the low and high CQF prevalence scenarios (Table 2). In the low CQF prevalence scenario, we assumed that only patients with a C. burnetii infection during the epidemic period were at risk for CQF. We divided them among high, middle, and low QF incidence areas using small geographic areas (4-digit postal code) and used incidence rates of QF notifications during the epidemic period for each incidence area. To adjust for underreporting, we multiplied the incidence rates by 12.6 (7). In the high CQF prevalence scenario, we assumed that all patients who seroconverted after the epidemic can develop CQF. For this scenario, we used larger geographic areas (3-digit postal code areas) and C. burnetii seroprevalences for each incidence area from the literature (24,25). In the second step, we estimated the risk for CQF using targeted screening studies for CQF conducted during or immediately after the epidemic (Appendix Table 4) (9–11,26,27). In the third step, we based the adjustment of the CQF prevalence from directly after the epidemic to the year of screening for the low CQF prevalence scenario on the reduction of CQF patients in the national CQF database over time (28). For the high prevalence scenario, we estimated this adjustment factor on the risk for CQF among patients with a heart valve disorder in studies conducted immediately after the outbreak (9,10) and a study conducted in 2016–2017 (29) (Appendix).
Detection Rate of Screening and Regular Care
We assumed a participation rate in the screening program of 50%, which is the lower bound of previous targeted screening programs for CQF in the Netherlands (10,27,30). The prevalence of CQF was assumed to be equal between participating and nonparticipating persons; hence, the participation rate affects only the number of CQF patients detected but not the cost-effectiveness of screening. We obtained sensitivity and specificity of ELISA from the literature; these values accounted for decreasing sensitivity over time after infection (31) (Appendix Table 5). CQF patients with high IgG against phase I were assumed to also have high IgG against phase II (C.C.H. Wielders, unpub. data [32]), which implies that all CQF patients test positive with ELISA. In the second screening round using IFA, patients with an IgG >1:512 against phase I were clinically evaluated. The detection rate of CQF in regular care is unknown; we used a detection rate of 80% for proven CQF, 50% for probable CQF, and 10% for possible CQF.
Outcome Probabilities
We estimated outcome probabilities using data from the national CQF database (Appendix Table 6). This database contains information about 439 CQF patients in the Netherlands, of whom 249 had proven, 74 had probable, and 116 had possible CQF (6). To estimate the effectiveness of screening, we stratified outcome data between CQF patients detected by regular healthcare (358 patients) and CQF patients detected by screening (78 patients). Proven CQF patients detected through screening had a 4.0 (95% CI 3.3–4.7) times lower risk for an early complication, 2.8 (95% CI 2.2–3.3) times lower risk for surgery, and 1.8 (95% CI 1.1–2.5) times lower risk for CQF-related death compared with proven CQF patients detected through regular care. The risk for a late complication did not differ significantly (risk ratio 0.7 [95% CI 0.1–1.4]) and was assumed to be equal between screening and regular care. For probable CQF patients, outcome probabilities were not significantly lower for screened patients than for patients identified through regular care. To avoid overestimation of the effect of screening, we conservatively assumed no effectiveness of screening for probable CQF patients and explored a scenario in which probable CQF patients benefit from screening in the sensitivity analysis. No clinical events were assumed in possible CQF patients (6). For undetected CQF patients, we used a higher risk for a late complication and death than for patients found through regular care.
QALYs and Costs
We estimated QALYs by multiplying the utility value associated with a certain health status by the years lived in that status. We obtained utility data for CQF-related complications from the literature (33–36) (Appendix Table 7). We applied a disutility for antimicrobial treatment (37,38). Average life expectancies of patients with premature CQF-related death were obtained from the national CQF database (6) (Appendix Table 8). For patients without premature CQF-related death, we assumed life expectancy to be half the life expectancy of a person at that age from the general population (39). We also obtained utility values for the general population from the literature (40) (Appendix).
We calculated costs in 2016 Euros (Appendix Table 9). Direct healthcare costs include costs of screening, diagnostic procedures, surgical procedures, antimicrobial drugs, specialist consultations, and lifelong costs of chronic complications. According to the national cost-effectiveness guideline (41), indirect healthcare costs (healthcare costs unrelated to CQF in life-years gained) should be taken into account, which we estimated using a prespecified tool (42). Because guidelines from other countries do not consider indirect healthcare costs, we show results without including indirect healthcare costs in the sensitivity analysis. Direct nonhealthcare costs include travel costs, and indirect nonhealthcare costs include productivity losses resulting from work absence (Appendix).
Cost-effectiveness and Sensitivity Analysis
We calculated the incremental cost-effectiveness ratio (ICER) of screening versus no screening by dividing the difference in costs by the difference in QALYs. We conducted a multivariate probabilistic sensitivity analysis using 10,000 simulations in which we varied a set of parameters at the same time within their uncertainty distributions. We conducted univariate sensitivity analyses, in which we varied several parameters one by one.
CQF Prevalence
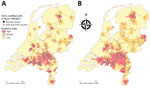
Depending on the size of the areas, 12% of the population (3-digit postal codes) or 16% of the population (4-digit postal codes) live in high QF incidence areas (Figure 2; Appendix Table 10). For the low CQF prevalence scenario, we estimated the number of C. burnetii infections at 42,143, resulting in 414 CQF patients directly after the epidemic and 102 CQF patients in the year of screening. For the high CQF prevalence scenario, the number of C. burnetii–infected persons was estimated to be 391,188, resulting in 3,842 CQF patients directly after the epidemic and 1,844 CQF patients in 2017. We also stratified the population by risk factor (Appendix Table 11). The prevalence of CQF varied substantially among risk groups and by residence area (Table 2); the highest prevalence occurred in cardiovascular risk patients living in high incidence areas (Appendix Table 12).
Clinical Impact
We determined the number of CQF patients and prevented clinical events for each subgroup (Table 3; Appendix Tables 13, 14). Most CQF-related events are prevented by screening of cardiovascular risk groups living in high incidence areas. At an assumed participation rate of 50%, 8 complications, 4 surgeries, and 2 premature deaths are prevented for the low CQF prevalence scenario and 105 complications, 54 surgeries, and 26 premature deaths for the high CQF prevalence scenario. Screening of immunocompromised patients or all adults >60 years of age living in high-risk incidence areas, or screening of cardiovascular risk groups in middle-incidence areas, also could prevent a substantial number of clinical events.
Cost-effectiveness
We determined the incremental costs, incremental QALYs, and ICERs for each subgroup (Table 3; Appendix Tables 15–17). The ICER of screening of cardiovascular risk groups living in high QF incidence areas was €31,737 per QALY for the low CQF prevalence scenario and cost-saving for the high CQF prevalence scenario. The next most cost-effective strategy would be screening of immunocompromised patients living in high incidence areas; ICERs were €66,145 per QALY for the low CQF prevalence scenario and €2,312 per QALY for the high CQF prevalence scenario. The ICER of screening for cardiovascular risk groups would increase substantially outside the high QF incidence area. For the high CQF prevalence scenario, the ICER increased from cost-saving to €12,929 per QALY in middle QF incidence areas and to €34,912 per QALY in low QF incidence areas. The ICER of screening for adults >60 years of age with an unknown risk factor living in high QF incidence areas was €679,136 per QALY in the low CQF prevalence scenario and €69,208 per QALY in the high CQF prevalence scenario. Screening of adults 18–59 years of age with an unknown risk factor was at least €8 million per QALY.
Sensitivity Analysis
We conducted a multivariate probabilistic sensitivity analysis (Figure 3; Appendix Figure 2). In the low CQF prevalence scenario, screening of cardiovascular risk patients living in high incidence areas had a 3.1% chance of an ICER <€20,000 per QALY and 92.5% chance of an ICER <€50,000 per QALY (Figure 3, panel A). In the high CQF prevalence scenario, screening had a 54.4% chance of being cost-saving and 100% chance of an ICER <€20,000 per QALY (Figure 3, panel B) for this subgroup.
The ICER was most sensitive to the lifetime costs of complications, the life expectancy of CQF patients, and the effectiveness of the screening program. For the low CQF prevalence scenario, the ICER varied from €17,561 to €63,449 per QALY (Figure 3, panel C). Adding the effectiveness of screening for probable CQF patients changed the ICER from €31,737 to €29,585 per QALY. Exclusion of indirect healthcare costs reduced the ICER to €25,681 per QALY (ICERs without the inclusion of indirect healthcare costs of other subgroups are shown in Appendix Table 18). Adding additional program costs of €11.36 per participant increased the ICER to €53,639 per QALY. For the high CQF prevalence scenario, the ICER remained cost-saving in most scenarios explored, and the highest ICER found was €1,903 per QALY (Figure 3, panel D).
We assessed the cost-effectiveness of a 1-time screening program for CQF in the Netherlands 7 years after a large QF epidemic. Cost-effectiveness varied substantially among areas and risk groups, and the results are highly sensitive to the prevalence of CQF. In a high CQF prevalence scenario, screening of cardiovascular risk patients living in high QF incidence areas during the epidemic was estimated cost-saving, whereas in a low CQF prevalence scenario the ICER was €31,737 per QALY for this subgroup. We found substantially higher ICERs for screening in areas with lower QF incidence during the epidemic or for screening of adults with an unknown risk factor for CQF.
A limitation is that the true prevalence of CQF 7 years after the epidemic is unknown. This prevalence can be affected by many factors, such as death from CQF or other causes, earlier diagnosis in regular care, and the background QF incidence after the epidemic. To account for uncertainty in CQF prevalence, we conducted a low and high CQF prevalence analysis. The estimated 42,000 new C. burnetii infections and 411 CQF patients during or after the epidemic low CQF prevalence scenario estimated correspond with previous estimates from the literature (7) or CQF patients included in the national database until May 2016 (6). However, these numbers are thought to be the absolute minimum. Only 23% of the proven CQF patients had a diagnosed acute QF episode (6), and a postmortem study among patients with a history of heart valve surgery in the epidemic area indicates that CQF possibly contributed to the death in 15% of the patients (9). The high CQF prevalence scenario could be the upper range because it does not account for preexisting immunity from before the epidemic. It is therefore likely that the true prevalence falls within the reported ranges.
Recent seroprevalence studies performed outside high QF incidence areas are lacking. Underreporting of QF could be higher in these areas because medical doctors are less familiar with QF symptoms (7). Furthermore, the geographic division between high, middle, and low QF incidence areas is arbitrary. Persons could be infected while traveling, and the extent to which extent farms with positive bulk milk samples contribute to disease spread is uncertain because 1 infected goat could yield a positive result.
The effectiveness of screening on the prevention of CQF-related complications and premature death is not well documented. We estimated the effectiveness by comparing outcome data between patients detected by screening and by regular care. We did this comparison separately for different CQF categories (proven, probable, or possible), but the effectiveness of screening can still be biased by uncontrolled confounders, such as age and presence of underlying conditions. The effectiveness of antimicrobial treatment for CQF has never been assessed in a randomized clinical trial. Surgery is known to have a positive effect on survival of CQF patients with vascular infection (3).
Our cost-effectiveness analysis is based on data from several sources in the Netherlands, such as spatial data on notifications of acute QF, seroprevalence data of C. burnetii infections, risk factor–specific probabilities of CQF given infection, and clinical data from a large number of CQF patients. However, combining data from different sources could also introduce biases when study populations do not exactly overlap or screening studies are conducted at different time-points.
Results of our study could also be relevant for other countries, where CQF also might be underreported. For instance, the seroprevalence of C. burnetii infection in the United States was estimated at 3.1% (43), representing millions of infections and potentially thousands of CQF cases, but no high numbers of CQF have been reported. An explanation may be that C. burnetii infections in the United States originate from cattle. The C. burnetii strains circulating in cattle differ from and are considered less pathogenic than the strains in small ruminants (3). In France, however, C. burnetii causes 5% of all endocarditis (44), and in Israel, C. burnetii infection was found in 9% of patients undergoing valve surgical procedure caused by endocarditis (45).
Cost-effectiveness is not the only criterion in deciding whether a screening program is justified (12). Screening for CQF is based on an antibody profile suggesting a chronic infection but cannot always be linked to a focus of infection (probable or possible CQF patients). Therefore, physicians must make difficult decisions about whether long-term antimicrobial treatment should be initiated when the outcome is uncertain and adverse events frequently occur. Raoult (46) has recently proposed alternative definition criteria for CQF from the consensus guideline in the Netherlands; these criteria could exclude most probable and possible CQF patients from follow-up but also may be less sensitive in the diagnosis of proven CQF (47).
When screening for CQF would be limited to subgroups for which screening is most cost-effective, a substantial proportion of CQF patients will remain undetected. Serologic follow-up for patients with acute QF is therefore recommended, even in absence of a risk factor for CQF (32). However, compliance with this recommendation was suboptimal during the epidemic (48), and many patients experience an acute infection asymptomatically or do not have the infection diagnosed. Alongside a standalone screening program, case finding could be implemented in regular care, in which the physician decides whether a patient should be screened according to a risk profile. Also, a combination of case-finding and screening programs among high-risk groups could be initiated; this approach has also been suggested for hepatitis B and hepatitis C (49).
Mr. de Boer is a health economist at the Center of Infectious Diseases of the National Institute for Public Health and the Environment, the Netherlands. His work focuses on the cost-effectiveness of preventive interventions against infectious diseases, such as vaccination and screening programs.
Acknowledgments
We thank Albert Jan van Hoek for providing useful comments on the methods and manuscript and Ben Bom for creating the Q fever incidence maps.
This study was financed from the regular budget of the Centre for Infectious Disease Control made available by the Ministry of Health, Welfare and Sport, project no. V/150207/17/RI.
References
- European Centre for Disease Prevention and Control. Risk assessment on Q fever. Stockholm: The Centre; 2010.
- Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–53.
- Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, et al. From Q Fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017;30:115–90.
- Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33:312–6.
- Kampschreur LM, Dekker S, Hagenaars JC, Lestrade PJ, Renders NH, de Jager-Leclercq MG, et al. Identification of risk factors for chronic Q fever, the Netherlands. Emerg Infect Dis. 2012;18:563–70.
- van Roeden SE, Wever PC, Kampschreur LM, Gruteke P, van der Hoek W, Hoepelman AIM, et al. Chronic Q fever–related complications and mortality: data from a nationwide cohort. Clin Microbiol Infect. 2019;25:1390–8.
- van der Hoek W, Hogema BM, Dijkstra F, Rietveld A, Wijkmans CJ, Schneeberger PM, et al. Relation between Q fever notifications and Coxiella burnetii infections during the 2009 outbreak in The Netherlands. Euro Surveill. 2012;17:20058.
- National Institute for Public Health and the Environment. Q fever [in Dutch] [cited 2017 May 23].
- Kampschreur LM, Oosterheert JJ, Hoepelman AI, Lestrade PJ, Renders NH, Elsman P, et al. Prevalence of chronic Q fever in patients with a history of cardiac valve surgery in an area where Coxiella burnetii is epidemic. Clin Vaccine Immunol. 2012;19:1165–9.
- Wegdam-Blans MC, Stokmans RA, Tjhie JH, Korbeeck JM, Koopmans MP, Evers SM, et al. Targeted screening as a tool for the early detection of chronic Q fever patients after a large outbreak. Eur J Clin Microbiol Infect Dis. 2013;32:353–9.
- Hagenaars JC, Wever PC, van Petersen AS, Lestrade PJ, de Jager-Leclercq MG, Hermans MH, et al. Estimated prevalence of chronic Q fever among Coxiella burnetii seropositive patients with an abdominal aortic/iliac aneurysm or aorto-iliac reconstruction after a large Dutch Q fever outbreak. J Infect. 2014;69:154–60.
- Wilson JMG, Jungner G. Principles and practice of screening for disease [cited 2017 Sep 1].
- Andermann A, Blancquaert I, Beauchamp S, Déry V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ. 2008;86:317–9.
- National Health Care Institute. Guideline for economic evaluations in healthcare [cited 2017 Dec 1].
- Statistics Netherlands. Population; gender, age, marital status and region, 1 January [in Dutch] [cited 2017 Dec 1].
- Vermeer-de Bondt PE, Schoffelen T, Vanrolleghem AM, Isken LD, van Deuren M, Sturkenboom MC, et al. Coverage of the 2011 Q fever vaccination campaign in the Netherlands, using retrospective population-based prevalence estimation of cardiovascular risk-conditions for chronic Q fever. PLoS One. 2015;10:
e0123570 . - van Hoek AJ, Andrews N, Waight PA, Stowe J, Gates P, George R, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65:17–24.
- Volksgezondheidenzorg.info. Reumatoid arthritis (RA) > numbers & context > current situation [in Dutch] [cited 2017 May 1]. ïde-artritis
- de Groof EJ, Rossen NG, van Rhijn BD, Karregat EP, Boonstra K, Hageman I, et al. Burden of disease and increasing prevalence of inflammatory bowel disease in a population-based cohort in the Netherlands. Eur J Gastroenterol Hepatol. 2016;28:1065–72.
- d’Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson-Stuttard J, Birks J, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–22.
- Pleumeekers HJ, Hoes AW, van der Does E, van Urk H, Hofman A, de Jong PT, et al. Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am J Epidemiol. 1995;142:1291–9.
- van der Hoek W, Wielders CC, Schimmer B, Wegdam-Blans MC, Meekelenkamp J, Zaaijer HL, et al. Detection of phase I IgG antibodies to Coxiella burnetii with EIA as a screening test for blood donations. Eur J Clin Microbiol Infect Dis. 2012;31:3207–9.
- Wegdam-Blans MC, Kampschreur LM, Delsing CE, Bleeker-Rovers CP, Sprong T, van Kasteren ME, et al.; Dutch Q fever Consensus Group. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J Infect. 2012;64:247–59.
- Pijnacker R, Reimerink J, Smit LAM, van Gageldonk-Lafeber AB, Zock JP, Borlée F, et al. Remarkable spatial variation in the seroprevalence of Coxiella burnetii after a large Q fever epidemic. BMC Infect Dis. 2017;17:725.
- Brandwagt DA, Herremans T, Schneeberger PM, Hackert VH, Hoebe CJ, Paget J, et al. Waning population immunity prior to a large Q fever epidemic in the south of The Netherlands. Epidemiol Infect. 2016;144:2866–72.
- Schoffelen T, Kampschreur LM, van Roeden SE, Wever PC, den Broeder AA, Nabuurs-Franssen MH, et al. Coxiella burnetii infection (Q fever) in rheumatoid arthritis patients with and without anti-TNFα therapy. Ann Rheum Dis. 2014;73:1436–8.
- Morroy G, van der Hoek W, Albers J, Coutinho RA, Bleeker-Rovers CP, Schneeberger PM. Population screening for chronic Q-fever seven years after a major outbreak. PLoS One. 2015;10:
e0131777 . - Buijs SB, Oosterheert JJ, Van Roeden SE, Kampschreur LM, Hoepelman AI, Wever PC, et al. Still new chronic Q fever cases diagnosed more than five years after a large Q fever outbreak [cited 2019 Sep 1].
- de Lange MMA, Scheepmaker A, van der Hoek W, Leclercq M, Schneeberger PM. Risk of chronic Q fever in patients with cardiac valvulopathy, seven years after a large epidemic in the Netherlands. PLoS One. 2019;14:
e0221247 . - Schoffelen T, Joosten LA, Herremans T, de Haan AF, Ammerdorffer A, Rümke HC, et al. Specific interferon γ detection for the diagnosis of previous Q fever. Clin Infect Dis. 2013;56:1742–51.
- Frosinski J, Hermann B, Maier K, Boden K. Enzyme-linked immunosorbent assays in seroprevalence studies of Q fever: the need for cut-off adaptation and the consequences for prevalence data. Epidemiol Infect. 2016;144:1148–52.
- Wielders CC, van Loenhout JA, Morroy G, Rietveld A, Notermans DW, Wever PC, et al. Long-term serological follow-up of acute Q-fever patients after a large epidemic. PLoS One. 2015;10:
e0131848 . - Franklin M, Wailoo A, Dayer MJ, Jones S, Prendergast B, Baddour LM, et al. The cost-effectiveness of antibiotic prophylaxis for patients at risk of infective endocarditis. Circulation. 2016;134:1568–78.
- Timmers TK, van Herwaarden JA, de Borst GJ, Moll FL, Leenen LP. Long-term survival and quality of life after open abdominal aortic aneurysm repair. World J Surg. 2013;37:2957–64.
- Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–51.
- Stouthard ME, Essink-Bot ML, Bonsel GJ, Barendregt JJM, Kramers PGN, van de Water HPA, et al. Disability weights for diseases in the Netherlands [cited 2019 Sep 1].
- Million M, Thuny F, Richet H, Raoult D. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis. 2010;10:527–35.
- World Health Organization. Global burden of disease 2004 update: disability weights for diseases and conditions. Geneva: The Organization; 2004.
- van Geldorp MW, Eric Jamieson WR, Kappetein AP, Ye J, Fradet GJ, Eijkemans MJ, et al. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg. 2009;137:881–6, 886e1-5.
- M Versteegh M. M Vermeulen K, M A A Evers S, de Wit GA, Prenger R, A Stolk E. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19:343–52.
- Versteegh M, Knies S, Brouwer W. From good to better: new Dutch guidelines for economic evaluations in healthcare. Pharmacoeconomics. 2016;34:1071–4.
- van Baal PH, Wong A, Slobbe LC, Polder JJ, Brouwer WB, de Wit GA. Standardizing the inclusion of indirect medical costs in economic evaluations. Pharmacoeconomics. 2011;29:175–87.
- Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR-03):1–30.
- Fournier PE, Casalta JP, Habib G, Messana T, Raoult D. Modification of the diagnostic criteria proposed by the Duke Endocarditis Service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–33.
- Maor Y, Sternik L, Orlov B, Rahav G, Keller N, Raanani E, et al. Coxiella burnetii endocarditis and aortic vascular graft infection: an underrecognized disease. Ann Thorac Surg. 2016;101:141–5.
- Raoult D. Chronic Q fever: expert opinion versus literature analysis and consensus. J Infect. 2012;65:102–8.
- Kampschreur LM, Wegdam-Blans MC, Wever PC, Renders NH, Delsing CE, Sprong T, et al.; Dutch Q Fever Consensus Group. Chronic Q fever diagnosis— consensus guideline versus expert opinion. Emerg Infect Dis. 2015;21:1183–8.
- Morroy G, Wielders CC, Kruisbergen MJ, van der Hoek W, Marcelis JH, Wegdam-Blans MC, et al. Large regional differences in serological follow-up of Q fever patients in the Netherlands. PLoS One. 2013;8:
e60707 . - Health Council of the Netherlands. Screening of risk groups for hepatitis B and C [In Dutch] [cited 2018 Sep 1].
Figures
Tables
Cite This ArticleOriginal Publication Date: 12/29/2019



































No hay comentarios:
Publicar un comentario