
A new DRUG TRIALS SNAPSHOT is now available.
Drug Trials Snapshots: PRETOMANID
PRETOMANID is a drug used for the treatment of lung tuberculosis (TB) in adults. It is to be used only when other drugs for the treatment of lung TB do not work (drug-resistant TB) or are not tolerated.
PRETOMANID is a tablet taken once a day for 26 weeks. It must be used in combination with two other antimicrobial drugs (bedaquiline and linezolid).
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
PRETOMANID
(Pre-TOH-mah-nid)
Mylan Laboratories Limited
Approval date: August 14, 2019
(Pre-TOH-mah-nid)
Mylan Laboratories Limited
Approval date: August 14, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PRETOMANID is a drug used for the treatment of lung tuberculosis (TB) in adults. It is to be used only when other drugs for the treatment of lung TB do not work (drug-resistant TB) or are not tolerated.
How is this drug used?
PRETOMANID is a tablet taken once a day for 26 weeks. It must be used in combination with two other antimicrobial drugs (bedaquiline and linezolid).
What are the benefits of this drug?
The trial showed that 95 out of 107 patients (89%) had no TB bacteria in the sputum six months after completing the treatment.
PRETOMANID is approved under FDA’s Limited Population Pathway for Antibacterial and Antifungal Drugs, which provides approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: PRETOMANID was similarly effective in men and women.
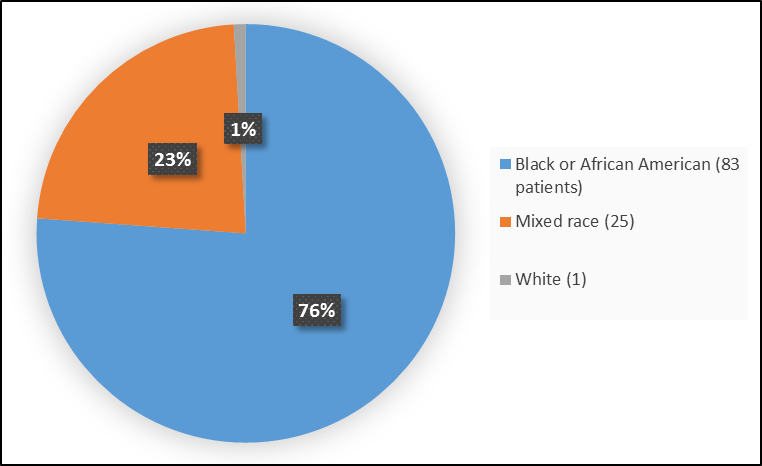
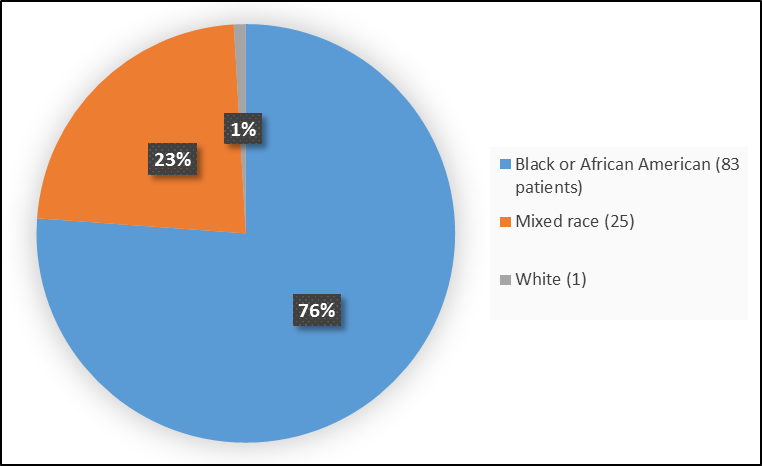
- Race: Most patients were Black. Differences in response to PRETOMANID among various race could not be determined.
- Age: PRETOMANID was similarly effective in adult patients below and above 30 years of age.
What are the possible side effects?
PRETOMANID taken together with bedaquiline and linezolid can cause serious side effects including:
- liver problems,
- bone marrow suppression (resulting in low blood counts),
- nerve damage,
- vision problems,
- changes in heart rhythm due to prolongation of heart electrical activity, and
- an acid build-up in the blood.
The most common side effects of PRETOMANID taken together with bedaquiline and linezolid include nerve damage, acne, anemia, nausea, vomiting, headache, and abnormal liver tests.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: Most patients were Black. Differences in side effects among various race could not be determined.
- Age: The occurrence of side effects was similar in patients below and above 30 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved PRETOMANID based on evidence from one clinical trial (NCT02333799) of 109 patients 17-60 years old for whom other drugs for the treatment of lung TB did not work (drug-resistant) or were not tolerated. The trial was conducted at 3 sites in South Africa. All 109 patients that provided data for evaluation of side effects (called the safety population) are presented in the charts below.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex (Safety Population)
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race (Safety Population)
FDA Review
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1 | 1 |
| Black or African American | 83 | 76 |
| Mixed | 25 | 23 |
FDA Review
Figure 3 summarizes the percentage of patients by age group in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
PRETOMANID was evaluated in one trial. Only adult patients for whom other drugs for the treatment of lung TB did not work (drug-resistant) or were not tolerated were included in the trial.
All patients in the trial received PRETOMANID together with bedaquiline and linezolid. All three drugs were taken by mouth, once daily for 6 months.
The benefit of this regimen was measured by the proportion of patients achieving a favorable response (no TB bacteria in the sputum) at 6 months after the end of treatment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.



































No hay comentarios:
Publicar un comentario