
Volume 25, Number 2—February 2019
Dispatch
Crimean-Congo Hemorrhagic Fever, Kosovo, 2013–2016
On This Page
Salih Ahmeti1, Lindita Berisha1, Bahrije Halili, Florim Ahmeti, Ronald von Possel, Corinna Thomé-Bolduan, Anett Michel, Simone Priesnitz, Emil C. Reisinger, Stephan Günther, Andreas Krüger, Kurtesh Sherifi, Xhevat Jakupi, Christoph J. Hemmer , and Petra Emmerich
, and Petra Emmerich
Abstract
During 2013–2016, a total of 32 patients were treated for Crimean-Congo hemorrhagic fever in Prishtina, Kosovo; 11 died. In the 11 patients who died, findings included viral loads >1 × 108.5/mL, lactate dehydrogenase >2,700 U/mL, bleeding, and impaired consciousness. Ribavirin therapy had no noticeable effect in this small patient sample.
Crimean-Congo hemorrhagic fever (CCHF), caused by an orthonairovirus of the Nairoviridae family, is usually transmitted by bites of Hyalomma sp. ticks. Case-fatality rates vary from 10% to 40%. Most cases are reported from the Balkans, the Middle East, and Asia (1), but CCHF virus is now also found in Hyalomma ticks in Spain, where human CCHF cases have occurred (2,3).
The earliest known case of CCHF in Kosovo was observed in 1954 (4). Since 1989, outbreaks have been seen every 4–5 years (5). CCHF is present in 50% of the Kosovar territory, especially the central and southwest, which is at low altitude, has a hot climate, and consists mainly of bush and farmland vegetation (6). The same study found a human CCHF seroprevalence in Kosovo of 24.3%, annual incidence of 0.49/100,000 population, and a case-fatality rate of 26.76% (for 1995–2009).
Incubation of CCHF may take up to 9 days. Signs and symptoms usually start 1–3 days after the tick bite and include sudden onset of fever, myalgia, neck stiffness, photophobia, vomiting, diarrhea, sore throat, agitation, and confusion; 2–4 days later, drowsiness and pain in the right upper quadrant of the abdomen may ensue. Other signs and symptoms include tachycardia, petechiae, and lymphadenopathy. Approximately 30% of patients die >5 days after disease onset or later, typically from bleeding or multiorgan failure (5).
We present a retrospective analysis of all 32 CCHF cases from the Infectious Diseases Hospital of Hasan Prishtina University (Prishtina, Kosovo) during May 2013–July 2016. We obtained a statement of approval from the Committee of Professional Ethics of the University Clinical Center of Kosovo (filed under no. 1555 on October 27, 2017).
We analyzed records of 32 patients with CCHF and excerpted demographic patient data, case history, symptoms, clinical signs, laboratory results, and outcome. Viral loads had been determined by reverse transcription PCR (RT-PCR) using a RealStar CCHFV RT-PCR Kit version 1.0 (Altona Diagnostics, http://www.altona-diagnostics.com). We used SPSS software (IBM, http://www.ibm.com/analytics/spss-statistics-software) for statistical analysis; p values of <0.05 by χ2 test or logistical regression (2-sided where applicable) were considered significant.
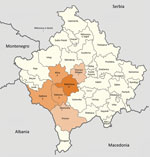
Figure 1. Municipalities (komunë) in Kosovo, showing number of patients with Crimean-Congo hemorrhagic fever in each municipality. Map was obtained from Wikimedia, where it is available to the public under the GNU Free...

Figure 2. Crimean-Congo hemorrhagic fever cases and deaths by age group and sex, Kosovo, 2013–2016.
Of the 32 patients, 27 were male and 5 female; 21 patients were from the municipality of Malisheva (district of Prizren) and 9 from municipalities west of Malisheva (Figure 1). Median age was 40.5 years (range 0–75 years) (Figure 2). Most patients were exposed to ticks during farming.
Eleven patients died, including 1 vertically infected female newborn; 21 survived. The median duration between initial symptoms and hospital admission was 2.5 days (range 0–7 days). Neither χ2 testing nor regression analysis showed a relationship between the duration of symptoms at hospitalization (or at the start of ribavirin) and outcome.
Of the 6 patients who did not receive ribavirin, 2 died. Of the 26 patients who received intravenous ribavirin, 9 died. Ribavirin had no effect on outcome; however, the sample size was small. The median duration between the start of symptoms and administration of ribavirin was 3.5 days (range 1–12 days). Univariate regression (odds radio [OR] 13.3, 95% CI 1.3–134; p = 0.028) and χ2 testing (p = 0.055) suggested a possible association between starting ribavirin <5 days after disease onset and an increased probability of death. However, this effect was not significant after controlling for viral load (p = 0.19).
Signs of central nervous system (CNS) impairment (coma, somnolence, fasciculations) and bleeding showed the strongest interdependence with clinical outcome (Table 1). A weaker interdependence was shown for jaundice, diarrhea, and hiccups. Among the laboratory parameters, a viral load >1 × 108.5copies/mL, lactate dehydrogenase (LDH) >2,200 U/mL, and leukocyte count >7,700 cells/µL were associated with death. We saw a nonsignificant trend toward interdependence between platelet counts <50,000/µL and death (p = 0.057). We found no association with outcome for aspartate aminotransferase, alanine aminotransferase, or other laboratory parameters (data not shown).
Of the clinical signs and symptoms, CNS impairment and bleeding predicted death. The death risk increased 35-fold (p = 0.003) in the presence of coma and 27-fold (p = 0.001) with somnolence; CNS impairment was present in all 11 patients who died. For fasciculations, we could not calculate an OR because no patient with fasciculations survived. Hemoperitoneum increased the death risk 16.6-fold (p = 0.004). If 4 of 5 bleeding signs (bleeding gums, hematemesis, melena, petechiae, ecchymoses) were present, the death risk increased 24-fold (p = 0.008). Diarrhea increased the death risk 7.2-fold (p = 0.023).
In multivariate analysis, the effects of bleeding, CNS involvement, and diarrhea on death risk were dependent on viral load and LDH levels. The effect of bleeding signs on death risk was independent of the effects of somnolence and of diarrhea. The effect of somnolence, but not of diarrhea, on death risk was independent of bleeding.
We saw no influence on death risk with other signs or symptoms (Table 2). Ribavirin therapy did not influence death risk (OR 1.059; p = 0.952) in the small sample size.
The strongest predictor of death was a viral load >1 × 108.5 viral copies/mL of serum (OR 80.0; p = 0.001), followed by an LDH >2,700 U/mL (OR 37.5; p = 0.006). The probability of death was also increased for leukocyte counts >7,700 cells/µL (OR 37.5; p = 0.006) and platelet counts <50,000/µL (OR 5.25; p = 0.041).
In multivariate analysis, viral loads >1 × 108.5 copies/mL and LDH levels >2,700 U/mL independently increased the probability of death (Table 2). However, the effect of leukocyte counts >7,700 cells/µL and platelet counts <50,000/µL did not persist when controlled for viral loads or LDH levels. No other routine laboratory parameters, including hemoglobin, creatine kinase, aspartate aminotransferase, alanine aminotransferase, prothrombin time, partial thromboplastin time, CCHF virus IgG, and CCHF IgM virus, predicted the outcome in the patients we analyzed.
Our results showed that the strongest clinical predictors of death from CCHF were bleeding and neurologic involvement. We saw somnolence or coma in all 11 fatal cases and bleeding in 9. In 2 patients, intracerebral bleeding could not be excluded because computed tomography or magnetic resonance imaging scans were not available. Therefore, the question whether all fatal neurologic complications in CCHF are caused by hemorrhage remains open.
Of laboratory parameters, the strongest predictors of death were viral load >1 × 108.5 viral genome copies/mL (OR 80) and LDH level >2,700 U/mL (OR 37.5). The predictive value of viral load has been described in Kosovo and Turkey (7,8). High viral loads predicted high LDH levels, which in turn predicted complications and death. CCHF virus infection may induce organ failure by causing apoptosis of several cell types of endothelial and parenchymal origin (9). Other factors probably contribute to CCHF pathology also.
We saw a higher case-fatality rate in Kosovo (34%) than that reported in Turkey (11%) (8). Although the virus clade circulating in Kosovo was probably introduced from Turkey in the 1970s, it is possible that the high genetic viral diversity found in fatal CCHF cases in Kosovo increases CCHF pathogenicity (10).
Our study did not detect any benefit of intravenous ribavirin for CCHF; however, even a recent Cochrane meta-analysis has not answered the question whether ribavirin is beneficial in CCHF (11). A murine treatment study suggests that ribavirin is insufficiently effective even when given before disease onset (12). To improve the prognosis for CCHF, new antiviral substances that effectively curb virus replication are needed.
Dr. Ahmeti is a professor of infectious diseases at Hasan Prishtina University and head of the Infectious Diseases Hospital of Prishtina, Republic of Kosovo. He has a special interest in Crimean-Congo hemorrhagic fever and other viral hemorrhagic fevers.
Acknowledgments
We thank the nurses of the Infectious Diseases Hospital of Prishtina, Kosovo, for their extraordinary dedication and contribution to the care of patients with CCHF.
Funding was provided by the German Ministry of Foreign Affairs (project no. ZMV-16 2513AA0277). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Vesenjak-Hirjan J, Punda-Polić V, Dobe M. Geographical distribution of arboviruses in Yugoslavia. J Hyg Epidemiol Microbiol Immunol. 1991;35:129–40.PubMed
- Ahmeti S, Kutllovci M, Bajrami M. Crimean Congo hemorrhagic fever in Kosova during 1995. Praxis Medica. 1996;39:11–6.
- Johnson S, Henschke N, Maayan N, Mills I, Buckley BS, Kakourou A, et al. Ribavirin for treating Crimean Congo haemorrhagic fever. Cochrane Database Syst Rev. 2018;6:CD012713.PubMed
- TUBS. Map of administrative divisions of Kosovo. 2013 Oct 22 [cited 2017 Nov 7]. https://commons.wikimedia.org/wiki/File:Kosovo,_administrative_divisions_(municipalities)_-_de_-_monochrome.svg
Figures
Tables
Cite This ArticleOriginal Publication Date: 1/7/2019
1These first authors contributed equally to this article.


































No hay comentarios:
Publicar un comentario