
| Clinical Trials | ||
| Updates from the National Cancer Institute | ||
| Clinical Trials News | ||
 | Immunotherapy Effective in Alveolar Soft Part Sarcoma People with advanced alveolar soft part sarcoma (ASPS), a rare type of soft tissue sarcoma, appear to benefit from immunotherapy with an immune checkpoint inhibitor called atezolizumab (Tecentriq), according to results from a small clinical trial. The phase 2 trial was conducted through NCI’s Experimental Therapeutics Clinical Trials Network. | |
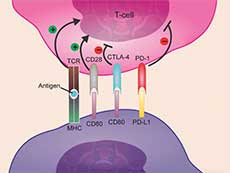 | Atezolizumab Approved for Initial Treatment of Metastatic Lung Cancer On December 6, 2018, the Food and Drug Administration (FDA) approved atezolizumab in combination with a standard three-drug regimen as initial treatment for advanced non-small cell lung cancer that does not have EGFR or ALK mutations. The approval is based on results from the phase 3 IMpower 150 clinical trial. | |
 | Interview with the Recently Retired CTEP Associate Director Jeff Abrams After 25 years with the Cancer Therapy Evaluation Program (CTEP), Jeff Abrams, M.D., retired from the federal government in November 2018. Dr. Abrams describes his career in oncology and some highlights in cancer research during his time at NCI, including the transformation of the NCI Cooperative Group Program into the National Clinical Trials Network. | |
| Find NCI-Supported Clinical Trials Use our search form to find a clinical trial or other research study that may be right for you or a loved one. | ||
| Clinical Trials Information for Patients and Caregivers | ||
| Steps to Find a Clinical Trial This step-by-step guide is intended to help people who are looking for clinical trials. The guide includes questions to ask about clinical trials and points to several resources for clinical trial information. | ||
| NIH Clinical Trials and You: The Basics The NIH Clinical Trials and You website is a resource for people who want to learn more about clinical trials. This page provides answers to common questions about taking part in a clinical trial. | ||
| NCI-Supported Clinical Trials That Are Recruiting Patients | ||
| Three-Drug Combination for Older Patients with Chronic Lymphocytic Leukemia This phase 3 trial is comparing standard therapy of ibrutinib and obinutuzumab with a three-drug combination of ibrutininb, obinutuzumab, and venetoclax for patients aged 70 or older with untreated chronic lymphocytic leukemia. The study will help determine if the addition of venetoclax improves progression-free survival, complete response rate, and overall survival. | ||
| Experimental Drug Therapy for Patients with Inoperable NF1-related GIST This phase 2 trial tests how well treatment with the experimental drug selumetinib works in patients with neurofibromatosis 1-related gastrointestinal stromal tumors (GIST) that cannot be removed by surgery. Researchers want to see if selumetinib stops the growth of these tumors without causing severe side effects. | ||
| Comparing Treatments for Residual Triple Negative Breast Cancer This phase 3 trial is comparing post-operative intravenous platinum-based chemotherapy and oral capecitabine for patients with residual triple negative breast cancer following neoadjuvant therapy. The study will help determine if one treatment is better than the other at preventing the disease from coming back. | ||


































No hay comentarios:
Publicar un comentario