Notes from the Field: Verona Integron-Encoded Metallo-Beta-Lactamase–Producing Pseudomonas aeruginosa Outbreak in a Long-Term Acute Care Hospital — Orange County, Florida, 2017
Weekly / June 1, 2018 / 67(21);611–612
Danielle Rankin, MPH1,2; Luz Caicedo, MPH1,2; Nychie Dotson, MPH2; Paige Gable3; Alvina Chu, MHS1 (View author affiliations)
View suggested citationOn July 5, 2017, one case of colonization with Verona integron-encoded metallo-beta-lactamase (VIM)–producing Pseudomonas aeruginosa was identified at a long-term acute care hospital (LTACH) in Orange County, Florida. VIM genes are capable of transferring among bacterial species (1); however, the mechanisms and frequency of resistance exchange is poorly understood (2). Thus, identification of colonization with VIM-resistant organisms is a sentinel event that warrants investigation and careful patient management. In response, the patient was placed on contact precautions and a facilitywide point prevalence survey was conducted (3). To detect colonization of VIM-producing P. aeruginosa, rectal swabs were collected from patients at the LTACH. The Florida Department of Health collaborated with the Tennessee Department of Health, the Southeast Regional Antibiotic Resistance Laboratory Network in Tennesee, and CDC to conduct antimicrobial resistance testing and genotyping.
During July 13–September 22, 2017, six additional patients at the LTACH screened positive for VIM-producing P. aeruginosa during three biweekly point prevalence surveys and an enhanced prospective surveillance system (Figure). The median length of stay at the LTACH among the seven colonized patients was 40.5 days (range = 13–150 days), and their median age was 60 years (range = 40–68 years); 57% were men. No patients reported hospitalizations or medical procedures outside the United States. Among the seven colonized patients, six had tracheostomy tubes (including three with current diagnoses of ventilator-dependent respiratory failure), six had decubitus ulcers, and four were receiving hemodialysis. Five patients had received antibiotic therapy before specimen collection, and one patient died approximately 1 month after colonization was detected. No cases of infection or complications associated with VIM-producing P. aeruginosa colonization have been reported at the LTACH. Pulsed field gel electrophoresis was conducted on four of the seven isolates; two had closely related (>90% similarity) patterns.
This investigation documents the first identification of VIM-producing P. aeruginosa in Florida. VIM-producing P. aeruginosa was first reported in Marseilles, France, in 1996 and has since been documented in health care–associated infections in several countries (4,5). Transmission can occur horizontally via hand carriage by health care personnel, through shared medical equipment, and through fomites (e.g., bedside tables, intravenous poles, bedside commodes, and sink drains) (4). Control measures include enhancing and reinforcing infection control processes and environmental disinfection. Measures taken in response to this outbreak investigation include 1) implementing an enhanced prospective surveillance program for P. aeruginosa isolates, 2) conducting infection control and response assessments (e.g., hand hygiene, personal protective equipment), 3) observing and reinforcing environmental cleaning practices, 4) implementing outbreak notification signage and patient discharge/transfer sheets, and 5) evaluating respiratory therapy processes.
Although carbapenem-resistant P. aeruginosa can be identified through routine culture and susceptibility testing, testing for mechanisms of resistance are not readily accessible. To detect the VIM-producing gene, additional antimicrobial resistance mechanism testing by polymerase chain reaction (PCR) must be conducted. Such testing is not routinely conducted at most clinical laboratories but is available now in all 50 states via CDC’s Antimicrobial Resistance Laboratory Network (ARLN).
Routine surveillance or PCR testing for antibiotic resistance mechanisms among P. aeruginosa is not practiced widely or uniformly; thus, the true incidence and prevalence of VIM-producing P. aeruginosa in the community and the risk for transmission among patients in health care facilities is unknown (6). Testing for common carbapenemases via the ARLN has the potential to better define the epidemiology of carbapenem-resistant P. aeruginosa resistance mechanisms as well as inform the response to control transmission. Reporting of organisms with high-priority antibiotic resistance mechanisms to public health authorities can inform regional infection control and containment practices.
Conflict of Interest
No conflicts of interest were reported.
Corresponding author: Danielle Rankin, Danielle.Rankin@flhealth.gov, 407-723-5053.
References
- Khorvash F, Yazdani M, Shabani S, Soudi A. Pseudomonas aeruginosa-producing metallo-β-lactamases (VIM, IMP, SME, and AIM) in the clinical isolates of intensive care units, a university hospital in Isfahan, Iran. Adv Biomed Res 2017;6:147. CrossRef PubMed
- Peter S, Oberhettinger P, Schuele L, et al. Genomic characterisation of clinical and environmental Pseudomonas putida group strains and determination of their role in the transfer of antimicrobial resistance genes to Pseudomonas aeruginosa. BMC Genomics 2017;18:859. CrossRef PubMed
- CDC. HAI and antibiotic use prevalence surveys. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. https://www.cdc.gov/hai/eip/antibiotic-use.html
- Castanheira M, Bell JM, Turnidge JD, Mathai D, Jones RN. Carbapenem resistance among Pseudomonas aeruginosa strains from India: evidence for nationwide endemicity of multiple metallo-β-lactamase clones (VIM-2, -5, -6, and -11 and the newly characterized VIM-18). Antimicrob Agents Chemother 2009;53:1225–7. CrossRef PubMed
- Edelstein MV, Skleenova EN, Shevchenko OV, et al. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect Dis 2013;13:867–76. CrossRef PubMed
- Lolans K, Queenan AM, Bush K, Sahud A, Quinn JP. First nosocomial outbreak of Pseudomonas aeruginosa producing an integron-borne metallo-β-lactamase (VIM-2) in the United States. Antimicrob Agents Chemother 2005;49:3538–40. CrossRef PubMed
 FIGURE. Colonization of patients at a long-term acute care hospital with Verona integron-encoded metallo-beta-lactamase–producing Pseudomonas aeruginosa, timing of point prevalence surveys and implementation of infection control, and isolate pulsed-field gel electrophoresis (PFGE) results — Orange County, Florida, July–September, 2017
FIGURE. Colonization of patients at a long-term acute care hospital with Verona integron-encoded metallo-beta-lactamase–producing Pseudomonas aeruginosa, timing of point prevalence surveys and implementation of infection control, and isolate pulsed-field gel electrophoresis (PFGE) results — Orange County, Florida, July–September, 2017
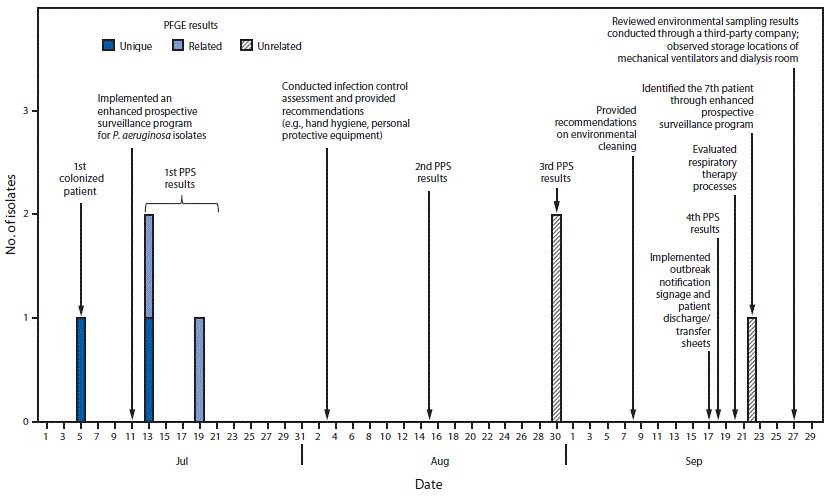
Abbreviation: PPS = point prevalence survey.
The figure above is combination bar chart and timeline showing the colonization of patients at a long-term acute care hospital with Verona integron-encoded metallo-beta lactamase-producing Pseudomonas aeruginosa, timing of point prevalence surveys and implementation of infection control, and isolate pulsed-field gel electrophoresis results, in Orange County, Florida during July–September, 2017.



































No hay comentarios:
Publicar un comentario