
FDA Approves Second CAR T-Cell Therapy for Lymphoma
May 22, 2018, by NCI Staff
On May 1, the Food and Drug Administration (FDA) approved the CAR T-cell therapy tisagenlecleucel (Kymriah) for adults with certain types of non-Hodgkin lymphoma, making it the second CAR T-cell therapy approved for lymphoma and the second FDA approval for this drug.
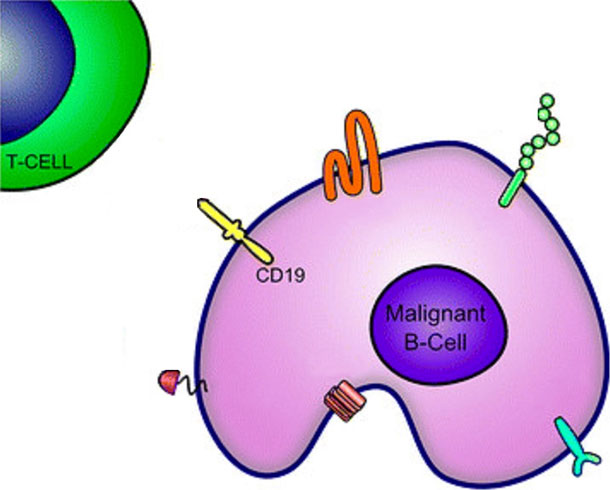
Last year, FDA approved another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), for the treatment of diffuse large B-cell lymphoma (DLBCL). CAR T-cell therapy is a type of immunotherapy that involves a one-time infusion of a patient’s own immune cells that have been genetically modified to recognize and attack cancer. Both tisagenlecleucel and axicabtagene ciloleucel target cells—both cancerous and normal—that express a molecule called CD19 on their surfaces.
Tisagenlecleucel was the first CAR T-cell therapy to receive FDA approval. It was approved in 2017 for the treatment of children and young adults with leukemia.
The new approval of tisagenlecleucel is for lymphoma—specifically DLBCL, high-grade B-cell lymphoma, and DLBCL that arises from follicular lymphoma—that has come back or gotten worse after prior treatment.
Tisagenlecleucel “definitely fills an important niche,” said Wyndham Wilson, M.D., Ph.D., head of the Lymphoma Therapeutics Section of NCI’s Center for Cancer Research. “But its impact, at least to begin with, will be limited” because the treatment is completely personalized and available only at certain cancer centers, Dr. Wilson added.
About 60% to 70% of people with DLBCL have lasting responses to their initial therapy. For people whose DLBCL does not respond (refractory disease) or comes back (relapses) after initial treatment, “the ability to cure [these patients] with conventional therapy is very, very limited,” Dr. Wilson explained.
Likewise, DLBCL that arises from follicular lymphoma can be hard to treat and the long-term prognosis for some of these patients is poor. Follicular lymphoma can sometimes transform into DLBCL after patients receive treatment for follicular lymphoma.
FDA’s new approval makes available another CAR T-cell therapy that “is likely to cure some patients [with DLBCL] who wouldn’t be cured with other available strategies,” Dr. Wilson said.
The JULIET Trial
The new approval was based on results from a phase 2 clinical trial, called JULIET, for people with relapsed or refractory DLBCL who previously had at least two systemic cancer treatments or a stem cell transplant. Novartis, the manufacturer of tisagenlecleucel, sponsored the trial.
Study participants received chemotherapy to eliminate their existing immune cells before receiving a single infusion of tisagenlecleucel.
Of the trial participants who could be evaluated, half had a response. Overall, 18% had a partial response, meaning their tumors shrank but not completely, and about one third of participants had a complete response, meaning all signs of the cancer disappeared. Complete responses lasted longer than partial responses.
Study participants need to be followed for a longer period to determine how long their responses last, Dr. Wilson noted.
In about one-quarter of the patients, tisagenlecleucel caused a severe inflammatory reaction known as cytokine release syndrome (CRS), a known side effect of CAR T-cell therapy. And 18% of patients had a neurological event, another common side effect of CAR T-cell therapy.
“These things can be managed, and we’re learning more about how to do that,” Dr. Wilson explained. “But that doesn’t mean they can’t be severe or life-threatening for some patients.”
FDA approved tisagenlecleucel with a Risk Evaluation and Mitigation Strategy, a drug safety program intended to help reduce the occurrence and severity of treatment side effects like CRS and neurological toxicity. Under the program, health care providers at facilities where tisagenlecleucel is offered must be specially trained to monitor and manage these side effects.
Other common side effects of tisagenlecleucel included infection, fever, diarrhea, nausea, fatigue, low blood pressure, edema, and headache.
In addition to the potential side effects, tisagenlecleucel is limited in several ways, Dr. Wilson pointed out. Not all people with DLBCL are eligible to receive the treatment or have access to one of the certified treatment centers. And it provides lasting benefits to only a minority of patients.
With FDA’s new approval, eligible patients with DLBCL now have two options for CAR T-cell therapy. For tisagenlecleucel and axicabtagene ciloleucel, the patient’s immune cells are modified in the same way. And the cost of both therapies is about the same. The only way to determine which of the two therapies is more effective would be to compare them head-to-head in a clinical trial, noted Dr. Wilson.






















.png)











No hay comentarios:
Publicar un comentario