
Volume 23, Number 5—May 2017
Research Letter
Clinical Manifestations of Punta Toro Virus Species Complex Infections, Panama, 2009
On This Page
Nathan D. Gundacker1, Jean-Paul Carrera1, Marlene Castillo, Yamilka Díaz, Jose Valenzuela, Ashutosh Tamhane, Brechla Moreno, Juan Miguel Pascale, Robert B. Tesh, and Sandra López-Vergès
Abstract
An investigation in Panama found that Punta Toro virus species complex (PTVs) may contribute to febrile illnesses with symptoms mirroring those of dengue fever. However, further studies are needed to determine if PTV infection causes only a mild disease or if it can have more serious manifestations in some patients.
Acute febrile illness in the New World tropics has a broad differential diagnosis largely dependent on locale and seasonal outbreaks. In Central America, most febrile illnesses have historically been attributed to dengue or malaria. However, recent evidence from Panama suggests varied differential diagnoses, including hantavirus, chikungunya virus, and Zika virus infection (1,2). In 2009, a dengue outbreak was reported in Panama City, Panama. The Gorgas Memorial Institute in Panama City tested dengue-negative samples from this outbreak for alphaviruses, flaviviruses, and phleboviruses and detected Punta Toro virus species complex (PTVs) in some samples. PTV (genus Phlebovirus, family Bunyaviridae), a member of the sand fly fever group, was initially described in humans in 1966 after being isolated from a soldier in Panama who had fever, headache, myalgia, and leukopenia (3). The phylogenetics of PTV have been thoroughly characterized (4–6), but our search of the literature did not reveal reports of other PTV cases in humans.
The symptoms of sand fly–associated phlebovirus infection vary, but most infections cause a mild febrile illness characterized by retroorbital headache, weakness, back pain, and leukopenia. However, infection with 2 other phleboviruses, mosquitoborne Rift Valley fever virus and tick-associated severe fever with thrombocytopenia syndrome virus, causes severe disease. Little is known regarding the signs, symptoms, and clinical course of PTV infection in humans.
During the 2009 investigation, the Gorgas Memorial Institute analyzed 4,852 samples from persons in Panama with suspected acute dengue; 1,667 (34.4%) of the samples were dengue-negative. We further analyzed 201 of these samples for phlebovirus (Technical Appendix[PDF - 282 KB - 3 pages]Table 1). In brief, we extracted viral RNA from the samples and evaluated it by using Phlebovirus genus–specific reverse transcription PCR (RT-PCR) based on the highly conserved L (large) gene that detects Toscana, Naples, Sicilian, Aguacate, Punta Toro, and Rift Valley fever viruses (7). We also screened samples using panflavivirus and panalphavirus RT-PCRs.
Of the 201 samples, 27 (13.4%) were RT-PCR–positive for phlebovirus. BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) nucleotide sequence comparison suggested all were PTVs; 1 was previously described as Cocle virus (4). We conducted phylogenetic analyses on 11 of the phlebovirus-positive samples, using a 482-nt sequence and MEGA7 software (8). An optimal maximum-likelihood tree confirmed the samples were PTVs; all samples from 2009 (GenBank accession nos. KY43355–KY435365) clustered together close to Cocle virus (Technical Appendix[PDF - 282 KB - 3 pages] Table 2) (4). Our attempts to isolate virus were unsuccessful. To determine if PTV had been previously detected, we tested 202 randomly selected dengue virus–negative samples from 2008; none was phlebovirus-positive.
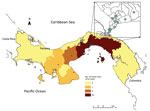
Figure. Distribution of confirmed Punta Toro species complex infections, Panama, 2009. Dots indicate cases. Inset shows enlargement of Panama City area.
Clinical data sheets were available for 92.6% (25/27) of the PTV-positive samples. After de-identifying the data, we entered it into a dataset. A control group consisted of 90 dengue virus–positive patients from 2009 who were frequency matched by age and randomly selected from available records. The PTV-positive case-patients were largely located in the Panama City metropolitan area (Figure). Case-patients and controls were compared primarily with regard to reported symptoms (Technical Appendix[PDF - 282 KB - 3 pages] Table 3). Case-patients were significantly less likely than controls to have exanthema (22% vs. 54%; odds ratio 0.23, 95% CI 0.08–0.66; p = 0.01). We found no major clinical differences between case-patients and controls with regard to other symptoms. No patients in either group had shock or hemorrhagic syndrome.
Febrile syndromes in the tropics are often treated empirically; clinical decisions are often made on the basis of epidemiologic information and concurrent outbreaks. In Central America, dengue fever and malaria are treated without confirmatory testing because testing is costly and time-consuming. However, an increasing number of agents responsible for causing febrile illnesses have been identified in recent years. The variety of clinical outcomes observed with hantavirus and dengue, chikungunya, and Zika virus infections underscores the need for more accurate diagnostics to differentiate between causative agents. Clinical decisions must rely on accurate diagnoses because symptomatology is not an accurate predictor of the true etiology of a febrile illness.
Our findings suggest that, in Panama, PTVs may be a major contributor to febrile illnesses with symptoms mirroring those of dengue fever. However, the clinical course and range of disease caused by PTVs are unknown. Prospective studies are needed to determine if PTV infection causes only mild disease or if it can have serious manifestations in some patients.
PTVs are assumed to be sand fly–borne, and sand flies are usually present in rural or forested areas (9). However, most cases of PTVs infection in Panama in 2009 were in urban and periurban areas, raising questions about the vector, the vector’s habitat, and the mode of virus transmission. Panama City, however, is home to two thirds of the country’s population and has improved healthcare infrastructure, which may explain the higher number of confirmatory tests from Panama City versus other areas of Panama and might result in a sampling bias. Despite these limitations, the recent Zika outbreak has shown the speed at which vectorborne diseases can spread and highlights the importance of detecting emerging viruses like PTVs.
Dr. Gundacker is an infectious disease fellow at the University of Alabama at Birmingham. His primary interest are the clinical description of febrile tropical infectious diseases, laboratory differential diagnosis of these diseases, and host–pathogen interactions. Mr. Carrera is an epidemiologist and virologist at Gorgas Memorial Institute. His primary research interests are ecology, evolution, and epidemiology of arthropodborne and zoonotic viruses.
Acknowledgments
We thank staff in the Department of Research in Virology and Biotechnology at the Gorgas Memorial Institute for Health Studies in Panama and in the Ministry of Health National Epidemiology Department for the surveillance data and outbreak response during 2009. We also thank Meghan Tipre for help creating the epidemiologic map.
This work was done in compliance with the Gorgas Bioethics Committee (1010/CBI/ICGES/15).
Funding was provided by the Panama Ministry of Economy and Finance (09.044.051 to S.L.-V. and 09.044.050 to J.M.P.); the Secretaría Nacional de Ciencia, Tecnología, e Innovación (SENACYT; FID-09-103 to J.-P.C); and Gorgas Memorial Institute, University of Alabama at Birmingham (to N.D.G.). J.M.P. and S.L.-V. are members of the Sistema Nacional de Investigación (SNI) of SENACYT in Panama.
References
- Armien B, Pascale JM, Munoz C, Lee SJ, Choi KL, Avila M, et al. Incidence rate for hantavirus infections without pulmonary syndrome, Panama. Emerg Infect Dis. 2011;17:1936–9. DOIPubMed
- Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med. 2016;374:601–4. DOIPubMed
- Tesh RB, Chaniotis BN, Peralta PH, Johnson KM. Ecology of viruses isolated from Panamanian phlebotomine sandflies. Am J Trop Med Hyg. 1974;23:258–69.PubMed
- Palacios G, Wiley MR, Travassos da Rosa AP, Guzman H, Quiroz E, Savji N, et al. Characterization of the Punta Toro species complex (genus Phlebovirus, family Bunyaviridae). J Gen Virol. 2015;96:2079–85. DOIPubMed
- Xu F, Chen H, Travassos da Rosa AP, Tesh RB, Xiao SY. Phylogenetic relationships among sandfly fever group viruses (Phlebovirus: Bunyaviridae) based on the small genome segment. J Gen Virol. 2007;88:2312–9. DOIPubMed
- Liu DY, Tesh RB, Travassos Da Rosa AP, Peters CJ, Yang Z, Guzman H, et al. Phylogenetic relationships among members of the genus Phlebovirus (Bunyaviridae) based on partial M segment sequence analyses. J Gen Virol. 2003;84:465–73. DOIPubMed
- Sánchez-Seco MP, Echevarría JM, Hernández L, Estévez D, Navarro-Marí JM, Tenorio A. Detection and identification of Toscana and other phleboviruses by RT-nested-PCR assays with degenerated primers. J Med Virol. 2003;71:140–9. DOIPubMed
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. DOIPubMed
- Valderrama A, Tavares MG, Andrade Filho JD. Anthropogenic influence on the distribution, abundance and diversity of sandfly species (Diptera: Phlebotominae: Psychodidae), vectors of cutaneous leishmaniasis in Panama. Mem Inst Oswaldo Cruz. 2011;106:1024–31. DOIPubMed
Figure
Technical Appendix
Cite This Article1These authors contributed equally to this article.






















.png)











No hay comentarios:
Publicar un comentario