
Volume 26, Number 4—April 2020
Research Letter
Plague Epizootic Dynamics in Chipmunk Fleas, Sierra Nevada Mountains, California, USA, 2013–2015
On This Page
Figures
Altmetric
Talisin T. Hammond1 , Kelly A. Liebman, Robert Payne, Helen K. Pigage, and Kerry A. Padgett
, Kelly A. Liebman, Robert Payne, Helen K. Pigage, and Kerry A. Padgett
Abstract
We describe Yersinia pestis minimum infection prevalence in fleas collected from Tamias spp. chipmunks in the Sierra Nevadas (California, USA) during 2013–2015. Y. pestis–positive fleas were detected only in 2015 (year of plague epizootic), mostly in T. speciosus chipmunks at high-elevation sites. Plague surveillance should include testing vectors for Y. pestis.
To better forecast vectorborne infection dynamics, characterizing disease cycles in both hosts and vectors is critical. The rate of infection of vector species can serve as a good indicator for risk during epizootic events, especially in areas with high human–wildlife overlap, but vectors are often poorly sampled. Yersinia pestis, the bacterium that causes plague, is carried by multiple flea species in western North America, where sciurids are often the primary reservoirs (1). Although human plague cases in this area are rare, in 2015, two cases were linked to exposures in Yosemite National Park, California, USA (2). In the investigation conducted to determine the source of these exposures, multiple Y. pestis–positive flea and rodent species were documented, and the lodgepole chipmunk (Tamias speciosus) was the host that was most frequently seropositive (2).
Plague surveillance in the western United States typically involves serologic testing of rodents and carnivores. Positive serologic results indicate prior plague activity. A Y. pestis–positive flea, however, indicates current plague transmission and is more likely to trigger control activities (3). Here, we sought to characterize Y. pestis infection in fleas of alpine (T. alpinus) and lodgepole (T. speciosus) chipmunks in Yosemite National Park and surrounding areas during 2013–2015. We focused on T. speciosus chipmunks because of their documented role in the 2015 epizootic (2) and on T. alpinus chipmunks because they co-occur with T. speciosus chipmunks (4) and little is known about their role in plague ecology. Our goals were to describe the proportion of T. speciosus and T. alpinus chipmunks harboring Y. pestis–positive fleas and the minimum infection prevalence of Y. pestis in fleas collected from these species across multiple sites and in years with and without known epizootic activity.
During June–October 2013–2015, we collected fleas from tagged chipmunks. Using a metal-pronged comb, we combed each animal 5 times down the dorsum, the tail, and each hind leg and placed collected fleas into vials containing 100% ethanol. These procedures were approved by the University of California, Berkeley, Animal Care and Use Committee (Berkeley, California, USA).
We identified key flea specimens (N = 122) (5–9) and then cleared, dehydrated, and mounted them on microscope slides (Denver Museum of Nature and Science accession nos. ZP.2000–176). For the remaining fleas, we microscopically observed and identified the species (5) using keys (6–9) and mounted some fleas as references. For each host, we pooled all conspecific fleas, which resulted in 162 pools (with 291 fleas total) from 121 T. alpinus chipmunks and 538 pools (with 1,096 fleas total) from 389 T. speciosus chipmunks (Appendix Table 1). We used molecular methods to detect Y. pestis DNA in flea pools (Appendix).
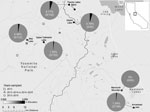
We found Y. pestis–positive fleas exclusively in 2015 at 5 of the 6 sites surveyed (Figure; Appendix Table 2). In 2015, 7.29% (14/192) of T. speciosus hosts carried >1 Y. pestis–positive flea. The minimum infection prevalence of Y. pestis in T. speciosus chipmunk–hosted fleas was 3.28% (assuming 1 positive flea per positive pool, 18 positive pools/548 total fleas in 280 pools tested). All 3 of the flea species (Ceratophyllus ciliatus mononis, Eumolpianus eumolpi, and E. eutamiadis) most commonly found on T. speciosus and T. alpinus chipmunks were found to be positive for Y. pestis (Appendix Table 1) (10). In 2015, a total of 5.13% (2/39) of T. alpinus hosts carried >1 Y. pestis–positive flea (Appendix Table 2). The infection prevalence (not minimum infection prevalence because each positive pool contained a single flea) of Y. pestis in T. alpinus chipmunk–hosted fleas was 2.47% (2 positive pools/81 total fleas in 50 pools tested). Unfortunately, these fleas were too damaged to identify morphologically, and molecular species identification was not possible.
Y. pestis–positive flea pools were detected at 5 of 6 high-elevation (2,650–3,200-m) study sites in 2015. Many of these sites are areas of high human activity, with popular hiking trails or established campgrounds. In 2015, plague risk assessments, including testing flea pools and rodent carcasses for Y. pestis DNA and rodent serology, also took place at lower elevation sites (1,778 ± 553 m) in and around the park; these surveys detected Y. pestis at 4 of 17 locations (2).
Altogether, our data indicate a dramatic shift in Y. pestis prevalence in fleas during a plague epizootic year in California. Our results support integrating flea testing, especially those at high-elevation sites, into regular surveillance.
Dr. Hammond is a postdoctoral fellow at the San Diego Zoo Institute for Conservation Research, Escondido, California, USA, and performed this work as a doctoral candidate at the University of California, Berkeley, California, USA. Her research interests focus on behavioral ecology, ecophysiology, disease ecology, and conservation.
Acknowledgments
We thank numerous field assistants, Mary Joyce Pakingan and Sabrina Horrack for assistance with laboratory work, and Jon Pigage for providing flea identification support.
T.T.H. was supported by a National Science Foundation graduate research fellowship during this work.
References
- Smith CR, Tucker JR, Wilson BA, Clover JR. Plague studies in California: a review of long-term disease activity, flea-host relationships and plague ecology in the coniferous forests of the Southern Cascades and northern Sierra Nevada mountains. J Vector Ecol. 2010;35:1–12.
- Danforth M, Novak M, Petersen J, Mead P, Kingry L, Weinburke M, et al. Investigation of and response to 2 plague cases, Yosemite National Park, California, USA, 2015. Emerg Infect Dis. 2016;22:2045.
- California Department of Public Health. California compendium of plague control. Appendix B: CDPH vector-borne disease section, plague surveillance risk evaluation form. 2016 Sep [cited 2019 May 22].
- Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 2008;322:261–4.
- Pigage HK, Pigage JC, Demboski JR. Siphonaptera from western North American chipmunks. Comp Parasitol. 2017;84:135–41.
- Hubbard CA. Fleas of western North America: their relation to the public health. Ames (IA): Iowa State College Press; 1947.
- Traub R, Rothschild M, Haddow JF. The Rothschild collection of fleas: the Ceratophyllidae: key to the genera and host relationships: with notes on their evolution, zoogeography and medical importance. London: Academic Press; 1983.
- Lewis RE, Lewis JH, Maser C. The fleas of the Pacific Northwest. Corvallis (OR): Oregon State University Press; 1988.
- Lewis RE, Jameson EW Jr. A review of the flea genus Eumolpianus Smit, 1983 with a discussion of its geographic distribution and host associations (Siphonaptera: Ceratophyllidae: Ceratophyllinae). J Vector Ecol. 2002;27:235–49.
- Hammond TT, Hendrickson CI, Maxwell TL, Petrosky AL, Palme R, Pigage JC, et al. Host biology and environmental variables differentially predict flea abundances for two rodent hosts in a plague-relevant system. Int J Parasitol Parasites Wildl. 2019;9:174–83.
Figure
Cite This ArticleOriginal Publication Date: 1/9/2020
1Current affiliation: San Diego Zoo Institute for Conservation Research, Escondido, California, USA.





















.png)












No hay comentarios:
Publicar un comentario