| ||
| Download the PDF of the podcast transcript (70KB) | ||
| Dr. Thomas Geisbert, a professor in the Department of Microbiology and Immunology at the University of Texas Medical Branch, discusses a vaccine to protect against Nipah virus disease. | ||
| Running time = 23:36 | ||
| Read the associated article in the June 2019 issue of the EID Journal Use of Single-Injection Recombinant Vesicular Stomatitis Virus Vaccine to Protect Nonhuman Primates Against Lethal Nipah Virus Disease — C. E. Mire et al. |

Volume 25, Number 6—June 2019
Research
Use of Single-Injection Recombinant Vesicular Stomatitis Virus Vaccine to Protect Nonhuman Primates Against Lethal Nipah Virus Disease
On This Page
Podcast
Article Metrics
Chad E. Mire, Joan B. Geisbert, Krystle N. Agans, Krista M. Versteeg, Daniel J. Deer, Benjamin A. Satterfield1, Karla A. Fenton, and Thomas W. Geisbert
Abstract
Nipah virus (NiV) is a zoonotic pathogen that causes high case-fatality rates (CFRs) in humans. Two NiV strains have caused outbreaks: the Malaysia strain (NiVM), discovered in 1998–1999 in Malaysia and Singapore (≈40% CFR); and the Bangladesh strain (NiVB), discovered in Bangladesh and India in 2001 (≈80% CFR). Recently, NiVB in African green monkeys resulted in a more severe and lethal disease than NiVM. No NiV vaccines or treatments are licensed for human use. We assessed replication-restricted single-injection recombinant vesicular stomatitis vaccine NiV vaccine vectors expressing the NiV glycoproteins against NiVB challenge in African green monkeys. All vaccinated animals survived to the study endpoint without signs of NiV disease; all showed development of NiV F Ig, NiV G IgG, or both, as well as neutralizing antibody titers. These data show protective efficacy against a stringent and relevant NiVB model of human infection.
Nipah virus (NiV) and Hendra virus (HeV) are highly pathogenic zoonotic agents in the paramyxovirus genus Henipavirus. Human case-fatality rates (CFRs) for these viruses historically have ranged from 40% to >90% (1). NiV is categorized as a Biosafety Level 4 (BSL-4) pathogen because of the substantial illness and death it causes and the lack of approved vaccines and therapeutic drugs for human use. In 2015, the World Health Organization listed NiV as a priority pathogen because it is likely to cause severe outbreaks and, in early 2018, placed NiV on the Blueprint list of priority diseases (). This WHO designation was bolstered because of a deadly NiV outbreak (CFR 89%) during spring 2018 in southwestern India, where NiV had not previously been reported (2).
Bats of the genus Pteropus are the primary reservoir in nature for NiV (3), but several other mammal species can be infected by NiV (4–7). Analysis of NiV genomes has identified 2 NiV strains responsible for outbreaks: Malaysia strain NiVM and Bangladesh strain (NiVB). NiVM caused the first identified outbreak of NiV during 1998–1999 in Malaysia and Singapore (≈270 persons infected; CFR ≈40%) (8,9) and perhaps was responsible for a 2014 outbreak in the Philippines (CFR ≈52%); however, this speculation is based on short genomic reads, so the NiV strain that caused this outbreak is not known (10). NiVB has caused repeated outbreaks in Bangladesh and northeastern India; outbreaks occurred almost every year during 2001–2015 (11–15). These NiVB outbreaks had higher CFRs, averaging ≈80% (14), and showed documented human-to-human transmission (11,16).
Eight experimental preventive candidate vaccines against henipaviruses have been evaluated in NiVM animal models: 1) canarypox and 2) vaccinia viruses encoding the NiVM fusion protein (F) or the NiVM attachment protein (G) that have shown protection against NiVM in hamsters and pigs (17,18); 3) a recombinant adeno-associated vaccine expressing the NiVM G protein that completely protected hamsters against homologous NiVM challenge (19); 4) recombinant vesicular stomatitis viruses (rVSVs) expressing the NiVM F protein or the NiVM G protein that had 100% efficacy in hamsters against NiVM (20); 5) rVSVs expressing the NiVB F protein or the NiVB G protein that completely protected ferrets from NiVM disease (21); 6) an rVSV expressing the Zaire ebolavirus (EBOV) glycoprotein (GP) and the NiVM G protein (rVSV-EBOV-GP-NiVG) that demonstrated efficacy in NiVM hamster (22) and African green monkey (Chlorocebus aethiops) (23) models; 7) a recombinant measles virus vector expressing the NiVM G protein that had efficacy in the NiVM African green monkey model (24); and 8) a recombinant subunit vaccine based on the HeV G protein (sGHeV) that completely protected small animals against lethal HeV and NiVM infections (25–27) and was efficacious in the robust African green monkey model of HeV (28) and NiVM infection (29). Of 8 vaccines, the sGHeV vaccine is furthest along in evaluation; it has received licensure as a veterinary vaccine for HeV in horses (Equivac HeV, Zoetis, ) in Australia and is being considered as a human vaccine against NiV. When tested against NiV, these 8 vaccine vectors have been tested only against NiVM infection in animal models, and although the antigenicity of these vaccines should not be a concern given that HeV G is an immunogen against NiVM infection, there are new data on the NiVB African green monkey model to consider as far as dose/regimen of vaccines.
NiVB infection in African green monkeys is more pathogenic than NiVM infection (30). This difference resulted in significantly reduced efficacy of antibody therapy because of temporal differences in viral load. Specifically, the human monoclonal antibody m102.4 that had been shown to completely protect African green monkeys against lethal NiVM disease when treatment was delayed until day 5 after virus exposure provided no protection when African green monkeys were challenged with NiVB and treated beginning at day 5 after virus challenge (30,31). Considering these new data, the current vaccines against NiV need to be evaluated for possible differences in dose/regimen against the more pathogenic NiVB infection in the robust African green monkey model. To assess single-dose vaccine efficacy, we evaluated the rVSV vaccine vectors expressing either the NiVB F or NiVB G proteins 28 days after a single-dose vaccination in the NiVBAfrican green monkey model, which most faithfully recapitulates human disease (5,30).
rVSV Vaccine Vectors and NiVB Challenge Stock
Statistical Analyses
Animal studies in BSL-4 and nonhuman primate work generally restrict the number of animals used, the volume of biological samples that can be obtained, and the ability to repeat assays independently and thus limit statistical analysis. Consequently, we present these data as the mean calculated from replicate samples, not replicate assays, and error bars represent SD across replicates (Figure 1, panels B, C, D).
Animal Ethics Considerations and Experiments
Healthy adult African green monkeys were handled in the animal BSL-4 containment space at the Galveston National Laboratory (Galveston, TX, USA). Research was approved under animal protocol 1310040 by the University of Texas Medical Branch Institutional Animal Care and Use Committee (Appendix).
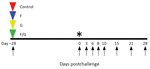
We used 10 adult African green monkeys weighing 3.5–6.0 kg in this study. One animal served as control (received GIn* rVSV-ΔG-GFP), and 3 animals per vaccine group received G* rVSV-ΔG-NiVB/F-GFP, G* rVSV-ΔG-NiVB/G-GFP, or rVSVΔG-NiVB/F/G. For vaccination, animals were anesthetized with ketamine and vaccinated with ≈107 PFU by intramuscular injection (day −28). Twenty-eight days after vaccination, the animals were exposed to ≈5 × 105 PFU of NiVB; the dose was equally divided between the intratracheal and the intranasal routes for each animal. Animals were monitored for clinical signs of illness (i.e., temperature, respiration quality, blood count, and clinical pathologic findings) at 0, 3, 6, 8, 10, 15, 21, and 28 days postchallenge (dpc).
NiVB Serum Neutralization Assays
We determined neutralization titers against NiVB using a conventional serum neutralization assay. In brief, we serially diluted serum 5-fold or 2-fold depending on magnitude of neutralization titers and incubated with ≈100 PFU of NiVB for 1 h at 37°C, as previously described (30).
RNA Isolation from NiVB-Infected African green monkeys
We isolated RNA from NiVB-infected animals as described previously (30). For viremia, we added 100 μL of blood to 600 μL of AVL viral lysis buffer (QIAGEN, ) for RNA extraction. For virus load in tissue, we stored ≈100 mg in 1 mL RNAlater (QIAGEN) for 7 d to stabilize RNA, removed the RNAlater completely, and homogenized tissues in 600 μL RLT buffer (QIAGEN) in a 2-mL cryovial using a tissue lyser (QIAGEN) and ceramic beads.
Detection of NiVB Load
We isolated RNA from blood or tissues and assessed it using primers and probe targeting the N gene and the intergenic region between N and P genes of NiVB for quantitative reverse transcription PCR (qRT-PCR). The probe used was 6FAM-5′CGT CAC ACA TCA GCT CTG ACA A 3′-6TAMRA (Life Technologies, ), as described previously (30).
Hematology and Serum Biochemistry
We assessed clinical pathology of NiVB-infected African green monkeys by hematology and serum biochemistry analysis as described previously (30). We performed the hematology assays using a laser-based hematologic analyzer (Beckman Coulter, ) and serum biochemistry analysis using a Piccolo point-of-care analyzer and Biochemistry Panel Plus analyzer discs (Abaxis, ).
Histopathology and Immunohistochemistry
We performed necropsies on all animals and collected tissue samples of all major organs. We performed histopathologic and immunohistochemical examination and analyses as described previously (30).
Immunization of African Green Monkeys and Measuring the Humoral Immune Response
Previously, single-injection, single-round replication rVSV vaccine vectors expressing the NiVB F or NiVB G proteins were described, characterized, and shown to be efficacious against NiVB challenge in ferrets (21). To assess the efficacy of these vectors in the NiVB African green monkey model, 4 groups of African green monkeys received a single intramuscular vaccination of rVSV vectors on day −28 (Figure 2). To analyze the antibody response to rVSV-ΔG-NiVB vaccinations, we assessed circulating antibodies for neutralization activity against NiVB before and after vaccination by using a 50% plaque-reduction neutralization titer (PRNT50) assay. All 4 groups had no detectable neutralizing antibody titers before vaccination (Table 1, day −28). On the day of challenge, the control animal (C-1) did not have detectable neutralizing antibody titers against NiVB, whereas all animals from the specific NiV protein vaccination groups (F, G, and F/G) had detectable neutralizing antibodies against NiVB (Table 1, day 0). Overall, the detectable neutralizing antibody response against NiVB reached a 1:640 dilution titer in the G and F/G groups and from 1:160 to 1:640 in the F group.
NiVB Challenge and Viral Load of Vaccinated African Green Monkeys
To determine the efficacy of the rVSV-ΔG-NiVB vectors against NiVB disease in African green monkeys, we challenged these animals by combined intratracheal and intranasal routes with a lethal challenge dose of NiVB on day 0 (Figure 1). All African green monkeys were closely monitored for up to 28 dpc for clinical signs of illness. The NiVB antigen vaccinated animals in the F (F-1–3), G (G-1–3), and F/G (F/G-1–3) groups showed no signs of clinical illness (Table 2) and were 100% protected against NiVB challenge (Figure 1, panel A), whereas the animal in the nonspecific vaccinated control group (C-1) exhibited clinical signs of disease (Table 2) and died of infection on day 8 (Figure 1, panel A). In addition, the control animal was the only NiVB-infected animal to have lymphopenia and serosanguinous nasal discharge during the course of disease (Table 2).
To determine the level of NiVB replication in animals after challenge, we assessed viral load by qRT-PCR on nasal and oral swab samples and whole blood samples (Figure 1, panels B–D). We detected NiVB genome equivalents (GEq) from nasal swab samples (Figure 1, panel B) in the control, F, G, and F/G groups. The following animals were positive for viral RNA: C-1 at 6 dpc; F-1 at 3 dpc; F-2 at 3, 6, and 10 dpc; G-1 at 3 dpc; G-3 at 6 dpc; F/G-1 at 3, 6, and 8 dpc; and F/G-3 at 3 dpc. At 6 dpc, when C-1 was positive for NiV RNA in nasal swab samples, the levels were >1 log higher than they were for the NiV-antigen vaccinated groups F, G, and F/G. Oral swab samples were negative for NiV RNA in all animals in the G-vaccinated group (Figure 1, panel C), and NiVB GEq were detected from oral swab samples in the control, F, and F/G groups. The following animals were positive for viral RNA: C-1 at 3 and 6 dpc, F-1 at 3 dpc, and F/G-1 at 3 and 6 dpc. Within these oral swab sample results, C-1 had NiV RNA levels up to 100-fold higher than the F and F/G animals that had positive oral swab samples (Figure 1, panel C). Unlike the results for swab samples, which represent tissues initially exposed to NiV, systemic and circulating NiVB GEq were not detected in whole blood from animals in the F, G, and F/G groups, whereas the control animal was positive in the blood sample from 6 dpc (Figure 1, panel D). The lack of systemic and circulating detection of NiVB RNA correlated with survival (Table 2; Figure 1, panel A).
Gross Pathologic, Histopathologic, and Immunohistochemical Analyses of NiVb-Infected African Green Monkeys
In the F, G, and F/G groups, we observed no gross pathologic findings at study endpoint. However, in the control animal that died of NiVBinfection, gross pathologic findings included serosanguinous pleural effusion, failure of all lung lobes to collapse with severe pulmonary hemorrhage and congestion, and multifocal to coalescing hemorrhage of the mucosal surface of the urinary bladder.
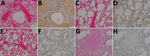
Lung sections examined from the control animal had moderate lymphoplasmacytic interstitial pneumonia characterized by a diffuse thickening of alveolar septae by moderate numbers of lymphocytes, plasma cells, polymerized fibrin, and edema fluid. The alveolar spaces were flooded by edema fluid, polymerized fibrin, foamy alveolar macrophages, and cellular debris. Endothelial syncytial cells were most apparent in medium- to small-caliber vessels (Figure 3, panel A). The animals in the F, G, and F/G groups had no major histologic findings in the lung sections (Figure 3, panels C, E, G). Immunohistochemical analysis revealed strong NiV antigen immunoreactivity within scattered alveolar macrophages and the endothelium of the alveolar septae and syncytial cells within medium to small caliber vessels in up to ≈75% of the examined pulmonary tissues (Figure 3, panel B). The lung sections of the F, G, and F/G groups were devoid of detectable NiV antigen (Figure 3, panels D, F, H).
Spleen sections from the control animal were depleted of lymphocytes in the multifocal follicular germinal centers within the splenic white pulp and were effaced by hemorrhage, fibrin, syncytial cell formation (Figure 4, panel A). Spleens from the F, G, and F/G groups had no major histologic findings (Figure 4, panels C, E, G). Immunohistochemical analysis of the spleen from the control animal revealed strong immunoreactivity for NiV antigen within the endothelium, syncytial cells, and scattered mononuclear cells in up to ≈50% of the examined splenic tissue (Figure 4, panel B), whereas the spleen sections of groups F, G, and F/G were devoid of detectable NiV antigen (Figure 4, panels D, F, H).
An important step in the preclinical development of a vaccine is efficacy testing in standards of animal models of disease. For NiV, the standard is the African green monkey model. Although the initial studies on the NiVM model in African green monkeys were reported as near uniformly lethal, data from several groups have revealed the model is not 100% lethal, depending on dose and route of infection (5,24,29–31,33,34). Combining the control animals from these studies, in which African green monkeys were challenged with various combinations of routes (e.g., intratracheal, intranasal, intraperitoneal, oral, small particle aerosol) at various doses, revealed that 18 (72%) of 25 animals died; however, most of the control animals were positive for circulating NiV RNA and had signs of clinical disease to varying degrees. Historically, our previous studies with the NiVB model has resulted in the deaths of all 14 control African green monkeys; the mean time to death was 7.14 days (Figure 2, panel A). We recently compared the pathogenesis of NiVM and NiVB strains in African green monkeys and observed that NiVB caused more pulmonary and splenic pathologic findings (30). We also observed the efficacy of time to treatment post-NiV challenge with a human monoclonal antibody m102.4 was shorter for NiVB-infected animals than for NiVM-infected animals (30). With these animal data in mind and the fact that NiVB has been responsible for most NiV outbreaks since 2002, we wanted to test our rVSV NiV vaccine vectors expressing NiVB F and G proteins as immunogens, which had 100% efficacy against NiVM challenge in ferrets (21), against NiVB challenge in African green monkeys.
In this study, we vaccinated 1 control African green monkey with a nonglycoprotein rVSV vector control, GInd* rVSV-ΔG-GFP, and 3 groups of 3 African green monkeys with NiV antigen vectors: GInd* rVSV-ΔG-NiVB/F-GFP, GInd* rVSV-ΔG-NiVB/G-GFP, or GInd* rVSV-ΔG-NiVB/F/G-GFP. The control animal, C-1, did not develop NiVB neutralizing antibodies by the day of challenge; had detectable circulating NiV RNA at 6 dpc; had clinical signs of NiV-mediated disease; and ultimately died of infection, showing typical NiV gross pathology and histopathologic findings. Conversely, the 3 rVSV NiV vaccine groups had animals in which detectable circulating NiV F, G, or F and G IgG developed, and circulating neutralizing antibody titers developed in all 3 groups by 28 days postvaccination. Each vaccine cohort had detectable NiVB RNA in nasal swab samples and only the F and F/G groups in oral swab samples, but none of the cohorts had any detectable circulating NiVB RNA throughout the course of the study. Consistent with the vaccine response from each cohort and the control of systemic spread of NiVB infection and control of NiV-mediated disease, all of the specifically vaccinated African green monkeys survived NiVB challenge.
The results of this study are similar to what we observed with these rVSV NiV constructs in the ferret model, which showed 100% protection regardless of the vaccine construct (21). Differences were that we found higher PRNT50 results for neutralizing antibody titers on day of challenge in this study and detected no circulating NiV RNA in the African green monkeys but did have detectable viral RNA at 6 dpc in the ferret study. Although we did not detect circulating viral RNA in the African green monkeys, the increase of neutralizing antibody titers at the study endpoint suggests sterilizing immunity was not achieved, and dosing or regimen will require further testing to reach sterilizing immunity with this single-round replication vaccine vector.
The single-round replication rVSV NiV vectors in this study and the replication competent rVSV-EBOV-GP-NiVG (23) are the only vaccine vectors to show 100% single-dose vaccine efficacy against NiV in the African green monkey model. Although both studies used this model, they differed in several ways. Our study used NiVB and challenged through the intratracheal and intranasal routes, whereas the other study used NiVM by the intratracheal route only (intratracheal challenge route used in initial model [5]). Here, we report detectable levels of NiV RNA in nasal swab samples at early times postchallenge, whereas the rVSV-EBOV-GP-NiVG study did not report any detectable NiV RNA in nasal swab samples. Whether these differences resulted from use of the intranasal route as part of the challenge cannot be determined here; however, neither study reported circulating levels of NiV RNA, indicating the prevention of systemic spread of NiV infection. Both studies reported the detection of circulating neutralizing antibodies on the day of challenge (28 [this study] and 29 days postvaccination). However, we reported on PFU reduction, and the rVSV-EBOV-GP-NiVG study reported on reduction of 200 50% tissue culture infectious dose in a tissue culture infectious dose assay, so the peak neutralizing titers at NiV challenge cannot be directly compared.
The PRNT50 titers we reported can be directly compared with the recombinant subunit sGHeV vaccine NiV study in African green monkeys that also was 100% efficacious (29), whereas we detected higher PRNT50 titers against NiV from the single injection of single-round replication vectors (from 160 to 640; Table 1) versus the PRNT50 titers 2 weeks after boost vaccination (from 28 to 379) for the recombinant subunit sGHeV vaccine. However, these lower titers most likely are due to the sGHeV vaccine being heterotypic because the PRNT50 titers against HeV in a similar African green monkey study were 640–1,280 on day of challenge (28). The development of neutralizing antibodies to the NiV glycoproteins after vaccination are important for protection, as highlighted by a single monoclonal antibody against the henipavirus G protein, m102.4, that is 100% protective against HeV, NiVM, and NiVB when administered at least 3 dpc (30,31,35).
In our study, the F cohort did not produce as consistent a neutralizing antibody titer response as did the G and F/G cohorts. Further analysis also revealed that, although no major changes occurred in hematologic and blood chemistry results for any of the vaccine cohorts, minor changes occurred in the F and F/G cohorts (Table 1). These data, taken together with the lack of detectable NiVB RNA in the oral swab samples of the G group, suggest the rVSV NiV G vector might be the better option among the 3 vaccine vectors.
In summary, we found that single-round replication rVSV vectors against NiVB provided 100% efficacy against NiVB challenge using a single-dose regimen. The rVSV vaccine platform has received attention recently because the replication-competent rVSV-ZEBOV GP vaccine vector against EBOV has now been given to >16,000 humans in clinical trials ranging from phase 1 to phase 3 and has been safe and efficacious (36); however, data for pregnant women and immunocompromised persons are not yet available. A single-round replication rVSV vaccine vector that is immunogenic and efficacious would have an attractive safety profile. Whether these single-round replication rVSV NiV vaccine vectors are as safe as the recombinant subunit sGHeV vaccine has yet to be determined, and the subunit vaccine has yet to be tested with a single-dose vaccine regimen. Although multidose vaccine regimens would be a potential strategy for laboratory and healthcare workers and for first responders in stable settings with defined risk for an NiV outbreak, an outbreak setting or a case of deliberate release of NiV would require rapid protection with a single administration of vaccine. The single-dose strategy was successfully enacted using a close-contact ring vaccination strategy with the rVSV-ZEBOV-GP vaccine at the end of the 2013–2016 EBOV epidemic (37–39). The strategy was so successful that it became the World Health Organization recommendation for future EBOV outbreaks and has recently been set into motion in the ongoing outbreak in the Democratic Republic of the Congo (40). Recent studies also suggest that the ring vaccination strategy for viruses such as EBOV (depending on transmissibility) that are endemic to countries that might not be able to afford a mass herd-immunity vaccination strategy might be more effective than mass vaccinations at controlling outbreaks (41). Further studies should examine the time to immunity of the GInd* rVSV-ΔG-NiVB/G in the NiVB African green monkey model because these data will be instrumental in providing information about whether this vaccine vector could be implemented in a ring vaccination strategy during future NiV outbreaks, such as the current one in India (2).
Dr. Mire is an associate professor in the Department of Microbiology and Immunology at the University of Texas Medical Branch–Galveston and the Galveston National Laboratory. His research focuses on understanding host–pathogen interactions of highly pathogenic RNA viruses.
Acknowledgments
We thank the staff of the University of Texas Medical Branch Animal Resources Center for animal husbandry, Robert W. Cross for assistance with the animal study, and Natalie Dobias for assistance with histologic processing. We thank Thomas G. Ksiazek for kindly providing the NiVB isolate used in this study.
This study was supported in part by National Institutes of Health (U01 AI082121 for research and UC7AI094660 for BSL-4 operations) and by funds provided to T.W.G. by the UTMB Department of Microbiology and Immunology.
References
- Wang L, Harcourt BH, Yu M, Tamin A, Rota PA, Bellini WJ, et al. Molecular biology of Hendra and Nipah viruses. Microbes Infect. 2001;3:279–87.
- Chatterjee P. Nipah virus outbreak in India. Lancet. 2018;391:2200.
- Halpin K, Hyatt AD, Fogarty R, Middleton D, Bingham J, Epstein JH, et al.; Henipavirus Ecology Research Group. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am J Trop Med Hyg. 2011;85:946–51.
- Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009;5:
e1000642 . - Geisbert TW, Daddario-DiCaprio KM, Hickey AC, Smith MA, Chan YP, Wang LF, et al. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS One. 2010;5:
e10690 . - Hooper P, Zaki S, Daniels P, Middleton D. Comparative pathology of the diseases caused by Hendra and Nipah viruses. Microbes Infect. 2001;3:315–22.
- Wong KT, Grosjean I, Brisson C, Blanquier B, Fevre-Montange M, Bernard A, et al. A golden hamster model for human acute Nipah virus infection. Am J Pathol. 2003;163:2127–37.
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–5.
- Uppal PK. Emergence of Nipah virus in Malaysia. Ann N Y Acad Sci. 2000;916:354–7.
- Ching PKG, de los Reyes VC, Sucaldito MN, Tayag E, Columna-Vingno AB, Malbas FF Jr, et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg Infect Dis. 2015;21:328–31.
- Gurley ES, Montgomery JM, Hossain MJ, Bell M, Azad AK, Islam MR, et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007;13:1031–7.
- Harcourt BH, Lowe L, Tamin A, Liu X, Bankamp B, Bowden N, et al. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg Infect Dis. 2005;11:1594–7.
- Hsu VP, Hossain MJ, Parashar UD, Ali MM, Ksiazek TG, Kuzmin I, et al. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis. 2004;10:2082–7.
- Lo MK, Lowe L, Hummel KB, Sazzad HM, Gurley ES, Hossain MJ, et al. Characterization of Nipah virus from outbreaks in Bangladesh, 2008-2010. Emerg Infect Dis. 2012;18:248–55.
- Luby SP, Hossain MJ, Gurley ES, Ahmed BN, Banu S, Khan SU, et al. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001-2007. Emerg Infect Dis. 2009;15:1229–35.
- Homaira N, Rahman M, Hossain MJ, Epstein JH, Sultana R, Khan MS, et al. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiol Infect. 2010;138:1630–6.
- Guillaume V, Contamin H, Loth P, Georges-Courbot MC, Lefeuvre A, Marianneau P, et al. Nipah virus: vaccination and passive protection studies in a hamster model. J Virol. 2004;78:834–40.
- Weingartl HM, Berhane Y, Caswell JL, Loosmore S, Audonnet J-C, Roth JA, et al. Recombinant nipah virus vaccines protect pigs against challenge. J Virol. 2006;80:7929–38.
- Ploquin A, Szécsi J, Mathieu C, Guillaume V, Barateau V, Ong KC, et al. Protection against henipavirus infection by use of recombinant adeno-associated virus-vector vaccines. J Infect Dis. 2013;207:469–78.
- Lo MK, Bird BH, Chattopadhyay A, Drew CP, Martin BE, Coleman JD, et al. Single-dose replication-defective VSV-based Nipah virus vaccines provide protection from lethal challenge in Syrian hamsters. Antiviral Res. 2014;101:26–9.
- Mire CE, Versteeg KM, Cross RW, Agans KN, Fenton KA, Whitt MA, et al. Single injection recombinant vesicular stomatitis virus vaccines protect ferrets against lethal Nipah virus disease. Virol J. 2013;10:353.
- DeBuysscher BL, Scott D, Marzi A, Prescott J, Feldmann H. Single-dose live-attenuated Nipah virus vaccines confer complete protection by eliciting antibodies directed against surface glycoproteins. Vaccine. 2014;32:2637–44.
- Prescott J, DeBuysscher BL, Feldmann F, Gardner DJ, Haddock E, Martellaro C, et al. Single-dose live-attenuated vesicular stomatitis virus-based vaccine protects African green monkeys from Nipah virus disease. Vaccine. 2015;33:2823–9.
- Yoneda M, Georges-Courbot M-C, Ikeda F, Ishii M, Nagata N, Jacquot F, et al. Recombinant measles virus vaccine expressing the Nipah virus glycoprotein protects against lethal Nipah virus challenge. PLoS One. 2013;8:
e58414 . - Mungall BA, Middleton D, Crameri G, Bingham J, Halpin K, Russell G, et al. Feline model of acute nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J Virol. 2006;80:12293–302.
- Pallister J, Middleton D, Wang LF, Klein R, Haining J, Robinson R, et al. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011;29:5623–30.
- Pallister JA, Klein R, Arkinstall R, Haining J, Long F, White JR, et al. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol J. 2013;10:237.
- Mire CE, Geisbert JB, Agans KN, Feng YR, Fenton KA, Bossart KN, et al. A recombinant Hendra virus G glycoprotein subunit vaccine protects nonhuman primates against Hendra virus challenge. J Virol. 2014;88:4624–31.
- Bossart KN, Rockx B, Feldmann F, Brining D, Scott D, LaCasse R, et al. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Sci Transl Med. 2012;4:
146ra107 . - Mire CE, Satterfield BA, Geisbert JB, Agans KN, Borisevich V, Yan L, et al. Pathogenic differences between Nipah virus Bangladesh and Malaysia strains in primates: implications for antibody therapy. Sci Rep. 2016;6:30916.
- Geisbert TW, Mire CE, Geisbert JB, Chan YP, Agans KN, Feldmann F, et al. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci Transl Med. 2014;6:
242ra82 . - Whitt MA, Geisbert TW, Mire CE. Single-vector, single-injection recombinant vesicular stomatitis virus vaccines against high-containment viruses. Methods Mol Biol. 2016;1403:295–311.
- Cong Y, Lentz MR, Lara A, Alexander I, Bartos C, Bohannon JK, et al. Loss in lung volume and changes in the immune response demonstrate disease progression in African green monkeys infected by small-particle aerosol and intratracheal exposure to Nipah virus. PLoS Negl Trop Dis. 2017;11:
e0005532 . - Johnston SC, Briese T, Bell TM, Pratt WD, Shamblin JD, Esham HL, et al. Detailed analysis of the African green monkey model of Nipah virus disease. PLoS One. 2015;10:
e0117817 . - Bossart KN, Geisbert TW, Feldmann H, Zhu Z, Feldmann F, Geisbert JB, et al. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci Transl Med. 2011;3:
105ra103 . - Suder E, Furuyama W, Feldmann H, Marzi A, de Wit E. The vesicular stomatitis virus-based Ebola virus vaccine: From concept to clinical trials. Hum Vaccin Immunother. 2018;14:2107–13.
- Gsell PS, Camacho A, Kucharski AJ, Watson CH, Bagayoko A, Nadlaou SD, et al. Ring vaccination with rVSV-ZEBOV under expanded access in response to an outbreak of Ebola virus disease in Guinea, 2016: an operational and vaccine safety report. Lancet Infect Dis. 2017;17:1276–84.
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. 2017;389:505–18.
- Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–66.
- Wise J. WHO is “cautiously optimistic” about Ebola ring vaccination programme in DRC. BMJ. 2018;361:k2388.
- Masterson SG, Lobel L, Carroll MW, Wass MN, Michaelis M. Herd immunity to Ebolaviruses is not a realistic target for current vaccination strategies. Front Immunol. 2018;9:1025.
Figures
Tables
Cite This ArticleOriginal Publication Date: 5/3/2019
1Current affiliation: Mayo Clinic, Rochester, Minnesota, USA.























.png)












No hay comentarios:
Publicar un comentario