
Volume 25, Number 9—September 2019
Research Letter
Characterization of Clinical Isolates of Talaromyces marneffei and Related Species, California, USA
On This Page
Figures
Article Metrics
Abstract
Talaromyces marneffei and other Talaromyces species can cause opportunistic invasive fungal infections. We characterized clinical Talaromyces isolates from patients in California, USA, a non–Talaromyces-endemic area, by a multiphasic approach, including multigene phylogeny, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, and phenotypic methods. We identified 10 potentially pathogenic Talaromyces isolates, 2 T. marneffei.
Talaromyces marneffei is a dimorphic fungal pathogen that causes focal or systemic infection in immunocompromised persons, primarily HIV-infected patients (1). Many cases have been reported in travelers returning from areas of Southeast Asia, southern China, and eastern India to which it is endemic. Other Talaromyces species also have been reported to cause invasive fungal infections, including T. amestolkiae (2), T. purpurogenus (3,4), and T. piceus (5,6). Talaromyces species are common in air, soil, and human habitats. Clinical laboratories in areas to which this fungus is not endemic often do not perform identification of T. marneffei and other Talaromyces species (2). Therefore, we devised a multiphasic approach for identifying T. marneffei and other potentially pathogenic Talaromyces species.
We conducted this study during 2018. Talaromyces isolates from 10 human specimens were submitted to the Microbial Diseases Laboratory (MDL), California Department of Public Health (Richmond, CA, USA), to rule out T. marneffei (Appendix). Temperature and pH are known to influence pigment production and colony morphology of Talaromycesspecies; therefore, growth characteristics were observed using 2 different culture media (Sabouraud dextrose agar, pH 5.6; and Sabouraud dextrose agar, Emmons, pH 6.9), incubated at 25°C and 30°C. Fungal DNA was extracted using a previously reported method (7). Talaromyces isolates were identified to species level using the internal transcribed spacer (ITS) region, partial β-tubulin gene (BenA), and partial RNA polymerase II largest subunit gene (RPB1) (8). The ITS and partial BenA and RPB1 sequences were used to search for homologies in GenBank and CBS databases (). Multigene phylogenetic analysis was conducted on the concatenated ITS–BenA–RPB1 nucleotide sequence alignment (Appendix). A blastn search () through the GenBank database, pairwise comparison alignment through the CBS database, or both showed 99%–100% homology for ITS, 97%–100% for BenA, and 91%–100% for RPB1 sequences with the best-matched sequences of known Talaromyces species isolates.
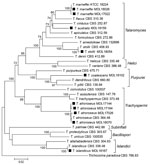
Phylogenetic analysis of the Talaromyces isolates showed 7 genetic clades, consistent with previous descriptions of the Talaromyces genera (9) (Figure). Species identification using a comparison of the ITS, BenA, and RPB1 sequences with existing sequences and multigene phylogenetic analysis identified T. marneffei (isolates MDL17022 and MDL18026), T. atroroseus (MDL17026, MDL17144, MDL17164, and MDL18070), T. islandicus (MDL18167), T. stollii (MDL18054), T. coalescens (MDL18102); and T. australis(MDL18159). The 2 T. marneffei isolates produced diffuse red pigment early, by 3 days of growth, on both medium types and at both incubation temperatures. T. australis and T. stollii isolates also produced red pigment by 3 days but with variations based on media or temperature. At 7 days of growth, the 4 T. atroroseus isolates also showed variable red pigment production (abundant, weak, and absent) (Appendix). Microscopically, most isolates showed biverticillate conidiophores and globose to fusiform conidia in unbranched chains. Both T. marneffei isolates were from HIV-positive patients. MDL17022 was from a blood sample of a 37-year-old man with a travel history to Southeast Asia; MDL18026 was from skin tissue of a 36-year-old man with no available travel history.
Using matrix-assisted laser desorption/ionizationtime-of-flight (MALDI-TOF) mass spectrometry, we generated main spectrum profiles (MSP) of Talaromyces species following Bruker’s custom MSP and library creation standard operating procedure (). We extracted proteins of Talaromyces isolates using the previously published National Institutes of Health (NIH) protocol (10). We analyzed Talaromyces spectra with MALDI Biotyper 4.1 software against combined databases of the Filamentous Fungi Library 2.0 (Bruker) and the NIH Mold Library (10), with and without inclusion of newly created MSPs of Talaromyces species (Appendix). The threshold for species identification was >1.9; for genus identification, >1.7.
Using the combined databases of Filamentous Fungi Library 2.0 (Bruker) and NIH Mold Library, we identified none of the isolates to species level; results showed either no identification or genus-level identification. However, when we expanded the combined database with the MDL Mold Library, we correctly identified all Talaromyces isolates to the species level with the best score >1.9. There were no ambiguous identification results; that is, the second-best matched species also had a high confidence score >1.9.
T. marneffei can be readily differentiated from other red pigment–producing Talaromyces species by yeast-like colony conversion at 37°C. However, many clinical laboratories no longer conduct yeast conversions. For those laboratories, yellow-green colonies producing red soluble pigment at ≈3 days on common fungal culture media at 25°C–30°C might indicate the need to further confirm T. marneffei. It is difficult to distinguish Talaromyces species only by macroscopic and microscopic examination. Multilocus sequencing, although confirmatory, might be too time-consuming and expensive for routine use. Therefore, we identified all Talaromyces isolates to species level by MALDI-TOF mass spectrometry by using an expanded database with well-characterized Talaromyces strains.
In conclusion, our results show that MALDI-TOF mass spectrometry is a good choice for rapid, less expensive primary identification of Talaromyces species and other medically important fungal pathogens. Species-level identification of Talaromyces isolates is clinically useful for treatment of patients with underlying conditions, such as immunodeficiency, cancer, advanced age, and immunosuppressive therapy.
Dr. Li is a research scientist and a certified public health microbiologist at the Microbial Diseases Laboratory, California Department of Public Health. Her primary research interests include molecular diagnosis of mycotic diseases, Valley fever, candidemia, antifungal susceptibility testing, mycobacteriology, metagenomics, and next-generation sequencing.
Acknowledgments
We thank Zenda Berrada for her support and helpful suggestions on this study.
The Microbial Diseases Laboratory at the California Department of Public Health provided funding for this study.
References
- Ustianowski AP, Sieu TP, Day JN. Penicillium marneffei infection in HIV. Curr Opin Infect Dis. 2008;21:31–6.
- Villanueva-Lozano H, Treviño-Rangel RJ, Renpenning-Carrasco EW, González GM. Successful treatment of Talaromyces amestolkiaepulmonary infection with voriconazole in an acute lymphoblastic leukemia patient. J Infect Chemother. 2017;23:400–2.
- Atalay A, Koc AN, Akyol G, Cakir N, Kaynar L, Ulu-Kilic A. Pulmonary infection caused by Talaromyces purpurogenus in a patient with multiple myeloma. Infez Med. 2016;24:153–7.
- Lyratzopoulos G, Ellis M, Nerringer R, Denning DW. Invasive infection due to penicillium species other than P. marneffei. J Infect. 2002;45:184–95.
- Santos PE, Piontelli E, Shea YR, Galluzzo ML, Holland SM, Zelazko ME, et al. Penicillium piceum infection: diagnosis and successful treatment in chronic granulomatous disease. Med Mycol. 2006;44:749–53.
- Horré R, Gilges S, Breig P, Kupfer B, de Hoog GS, Hoekstra E, et al. Case report. Fungaemia due to Penicillium piceum, a member of the Penicillium marneffei complex. Mycoses. 2001;44:502–4.
- Romanelli AM, Fu J, Herrera ML, Wickes BL. A universal DNA extraction and PCR amplification method for fungal rDNA sequence-based identification. Mycoses. 2014;57:612–22.
- Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, et al. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol. 2011;70:159–83.
- Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol. 2014;78:175–341.
- Lau AF, Drake SK, Calhoun LB, Henderson CM, Zelazny AM. Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry.J Clin Microbiol. 2013;51:828–34.
Figure
Cite This ArticleOriginal Publication Date: 7/30/2019
1Current affiliation: New York State Department of Health, Albany, New York, USA.
2Current affiliation: Hawaii State Department of Health, Pearl City, Hawaii, USA.





















.png)












No hay comentarios:
Publicar un comentario