
Volume 25, Number 2—February 2019
Dispatch
Differential Shedding and Antibody Kinetics of Zika and Chikungunya Viruses, Brazil
On This Page
Fernando A. Bozza1, Andres Moreira-Soto1, Alexandra Rockstroh, Carlo Fischer, Alessandra D. Nascimento, Andrea S. Calheiros, Christian Drosten, Patrícia T. Bozza, Thiago Moreno L. Souza, Sebastian Ulbert, and Jan Felix Drexler
Abstract
In seroconversion panels obtained from patients from Brazil, diagnostic testing for Zika virus infection was improved by combining multiple antibody isotypes, techniques, and antigens, but sensitivity remained suboptimal. In contrast, chikungunya virus diagnostic testing was unambiguous. Recurrent recent arbovirus infections suggested by serologic data and unspecific symptoms highlight the need for exhaustive virologic testing.
In 2013, Zika virus and chikungunya virus (CHIKV) emerged in Latin America (1,2). Their overlapping symptoms challenge accurate diagnosis on the basis of clinical manifestations (3). Direct Zika virus and CHIKV detection is limited to the acute phase of infection (4). Serologic detection of Zika virus–specific antibodies is hampered by low specificity and sensitivity of tests because of immune responses elicited by prior infection with other endemic flaviviruses (e.g., dengue virus [DENV]) (5,6). In addition, lack of adequate specimens limits studies evaluating the performance of diagnostic tests in tropical areas (7,8). To evaluate these challenges, we analyzed virus shedding and antibody responses over time in patients in Brazil sampled during the 2016 Zika virus and CHIKV outbreaks.
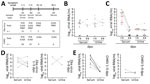
Figure 1. Overview of diagnostic testing and shedding dynamics for Zika virus and CHIKV among patients in Brazil, 2016. A) Timeline of sampling and number of samples for each test. B, C) Zika...
We prospectively sampled patients in 4 time points up to 90 days post–symptom onset (dpo) (Table 1; Figure 1, panel A; Appendix). The cohort comprised 15 patients with acute Zika virus infection (5 male, 10 female; median age 39.0 years [interquartile range 31.0–44.0 years]) and 18 patients with acute CHIKV infection (10 male, 8 female; median age 39.0 years [interquartile range 31.0–57.3 years]), determined by detection of viral RNA in blood or urine 1–9 dpo (Appendix Figures 1, 2). All Zika virus belonged to the Asian lineage (2), and all CHIKV to the East/Central/South African lineage, according to envelope-based typing.
At enrollment, Zika virus patients most frequently reported fever, rash, and arthralgia (80% each), and CHIKV patients most frequently reported arthralgia (100%), fever (89%), and myalgia (89%) (Table 2). No co-infection with Zika virus, CHIKV, or DENV was detected by real-time reverse transcription PCR (rRT-PCR). However, serologic analyses found that 4 (27%) Zika virus–infected patients also had CHIKV IgM at enrollment, and 1 (7%) had DENV IgM (Appendix Table 1, Figure 3). Similarly, 3 (17%) CHIKV-infected patients had Zika virus IgM, and 4 (22%) CHIKV-infected patients had DENV IgM at enrollment (Appendix Figure 4). We cannot exclude the possibility of cross-reactivity between Zika virus–specific and DENV-specific antibodies because 2 CHIKV patients simultaneously showed Zika virus and DENV IgM in an envelope-based ELISA (Appendix Table 2). Seventy-nine percent of Zika virus and 83% of CHIKV patients showed serologic evidence for past DENV infection at enrollment (Appendix Figures 1, 2). Thus, recent infections with heterologous arboviruses might bias attributing infection-specific symptoms for Zika virus and CHIKV.
Consistent with previous studies (4,9), Zika virus loads in serum and urine were low up to 9 dpo (≈104 RNA copies/mL) (Figure 1, panel B), whereas CHIKV loads were high ≈100-fold higher (≈106 RNA copies/mL) (Figure 1, panel C). However, unlike with Zika virus, CHIKV loads decreased significantly (p<0.001 by t test) from 5 dpo onward, and viral loads in urine were consistently low (Figure 1, panels D, E).

Figure 2. Zika virus and CHIKV antibody dynamics among samples from patients in Brazil, 2016. A) Percentage seroconversion for markers of acute infection with Zika virus and CHIKV (IgM NS1–based Zika virus ELISA,...
Next, to assess the antibody kinetics of Zika virus and CHIKV, we measured antibody responses over time by commercial and in-house serologic tests. In a widely used nonstructural (NS) protein 1 antigen-based ELISA, Zika virus IgM seroconversion was low (33% [5/15]), whereas CHIKV IgM seroconversion was 100% using an envelope-based ELISA (p<0.0001 by Fisher exact test) (Figure 2, panel A; Appendix Tables 1, 2). Use of an in-house envelope-based ELISA increased the Zika virus IgM detection rate to 50% (7/14), and use of a commercially available μ-capture ELISA increased it to 43% (6/14) (Figure 2, panel A). Despite differential sensitivity, concordant results from different assays suggest comparable specificity of IgM detection (Appendix Table 1). The use of NS1-based IgA as a marker of acute infection increased the detection rate to 53% (8/15) over that of the NS1-based IgM ELISA. All IgM-positive patients also showed IgA, which increased during acute and subacute phases of infection and decreased during convalescence (Figure 2, panel B; Appendix Figure 3). This finding supports the usability of IgA-based serologic methods as an alternative or additional marker to IgM-based methods to detect acute Zika virus infection. The detection rate increased 2-fold when we used NS1-based IgA from when we used NS1-based IgM 5–9 dpo, suggesting that IgA could be used at later stages of infection (Appendix Figures 1, 5). Our findings indicate that serologic detection of acute Zika virus infection can be improved ≈2-fold by use of different antibody classes and antigens but remains poorly sensitive in flavivirus-endemic areas.
All Zika virus–infected patients showed IgG responses across the 4 time points in >1 assay (Figure 2, panels C, D). Plaque reduction neutralization tests (PRNTs) were negative for 2 of 14 rRT-PCR–confirmed Zika virus cases detected by NS1-based IgG ELISA. Without rRT-PCR confirmation, these cases would have been classified false positive (Appendix Table 1). This observation might be explained by differential sensitivity of PRNT and ELISA (10) or false-positive results of the Zika virus NS1-based ELISA in secondary flavivirus infections (6). Similarly, the antibody kinetics of Zika virus NS1-based IgG, envelope-based IgG, and PRNT suggested either relatively early IgG seroconversion or cross-reactivity during acute stages of infection resulting from unspecific immune responses against other flaviviruses (11) (Figure 2, panel D). In contrast, CHIKV IgG seroconversion occurred at later stages (Figure 2, panel D; Appendix Figure 5), possibly associated with strong and long-lasting CHIKV-specific IgM responses (Appendix Figure 4).
We provide pivotal data on Zika virus and CHIKV diagnostic challenges in a Latin American setting. Limitations of our study include the relatively small number of patients, sampling at heterogeneous dpo and heterogeneous numbers of samples per dpo, and lack of acutely DENV-infected patients to assess test specificity. The strengths of our study include rRT-PCR–confirmed infections, waiving the need to define serologic assays prone to cross-reactivity as standards, sampling during Zika virus and CHIKV outbreaks (1,2), sequential sampling of patients up to 90 dpo, use of multiple antigens and immunoglobulin classes, and the combination of molecular and serologic testing methods.
Our data suggest reliable diagnostic testing for acute CHIKV infections by IgM detection from 5 dpo onward. This finding might enable waiving labor-intense and costly molecular protocols in many patients, minimizing costs for public health systems and cohort studies investigating arbovirus pathogenesis. However, reliability of CHIKV serologic diagnostic tests must be reevaluated for co-circulating genotypes (12) and for the antigenically related Mayaro virus (13) if it emerges in Latin America.
The difficulties of adequately diagnosing Zika virus infections in areas to which it is endemic have major implications for public health. Reliable testing for flaviviruses in such areas will be key for epidemiologic studies on Zika virus and assessments of the safety of flavivirus vaccination programs, as illustrated by more severe dengue infections in DENV-seronegative individuals who received a live attenuated dengue vaccine (14).
For pregnant women and couples intending pregnancy, accurate diagnosis of acute or past Zika virus infection is crucial. The steep increase in requests for abortion in Latin America illustrates the effect of the Zika virus outbreak on reproductive medicine (15).
Our results highlight that definite exclusion of acute Zika virus infections is challenging in a considerable proportion of patients. However, although limited by a small number of samples, our data highlight the attainability of more accurate Zika virus diagnostic testing by combining molecular and serologic tests using different antibody classes, antigens, and methods and by monitoring an increase of IgG titers in follow-up serum samples. Our data will help clinicians and health authorities build reliable diagnostic algorithms for Zika virus and CHIKV and highlight that exhaustive testing of arboviral infections is required for attributing frequencies of infection-specific symptoms.
Dr. F.A. Bozza is a senior scientist and head of the Laboratório de Medicina Intensiva, Fundação Oswaldo Cruz Rio de Janeiro. His research focuses on the host immune response and metabolic adaptation to severe infections.
Acknowledgments
We thank Ignacio Postigo-Hidalgo, Jens Miguel Warnecke, and Thiago Carvalho for technical assistance.
This work was supported by the European Union’s Horizon 2020 Research and Innovation Programme through the ZIKAlliance project (grant agreement no. 734548), the German Centre for Infection Research through the ZIKApath project, by the Conselho Nacional de Desenvolvimento e Pesquisa (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
References
- Steinhagen K, Probst C, Radzimski C, Schmidt-Chanasit J, Emmerich P, van Esbroeck M, et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill. 2016;21:30426. DOIPubMed
- Hassing RJ, Leparc-Goffart I, Tolou H, van Doornum G, van Genderen PJ. Cross-reactivity of antibodies to viruses belonging to the Semliki forest serocomplex. Euro Surveill. 2010;15:19588.PubMed
- World Health Organization. Updated questions and answers related to the dengue vaccine Dengvaxia® and its use [cited 2018 Jan 30]. http://www.who.int/immunization/diseases/dengue/q_and_a_dengue_vaccine_dengvaxia_use/en/
Figures
Tables
Cite This ArticleOriginal Publication Date: 1/7/2019
1These authors contributed equally to this article.


































No hay comentarios:
Publicar un comentario