
Studying the Redox Chemistry of Heam Proteins In Vivo using a Raman Microscope
Shutterstock | ibreakstock
Raman spectroscopy is considered to be sensitive to the existence of haem proteins and is perfect for studying their redox biology, without the requirement for staining or isolation.
The redox of haem proteins is closely associated with their protein functions – electron transport, oxygen transport and storage, and scavenging of free radicals. By using Raman spectroscopy to explain redox states within biological systems, researchers will be able to study redox dynamics and its effects on diseases and health regulation.
Images produced from Raman spectra can be employed for visualizing the spatial relationships, distribution, spin and redox states of haem proteins within biological systems. It is possible to process the data in order to reveal a multitude of information.
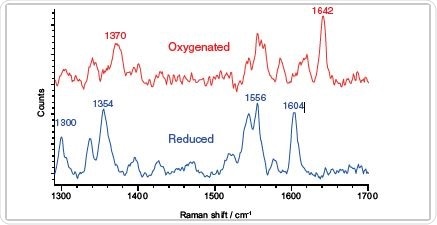
Clearly define redox state according to the Raman features – oxygenated and reduced myoglobin.
Identify Haem Protein Type and their Electronic States
- Detect haem proteins by their Raman spectra
- Establish their redox and spin states by Raman band positions
- Resonance Raman spectroscopy with visible wavelengths can precisely improve the signal intensities of haem protein bands, providing high sensitivity
- Isolation of protein is not needed
- Analyze haem proteins within tissues (whole organ/section) and cells (live/fixed)
Reveal Haem Protein Information Within Biological Systems In Situ
- Raman imaging offers in depth (sub-micrometer) spatial information
- Clearly show the location of haem proteins present in the sample
- Provide information on the relationship between their distribution, organelle, protein and cell functions
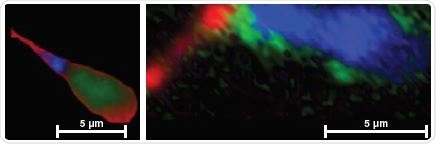
In cells: analyze haem proteins’ distribution, levels and redox state in situ. Reduced cytochrome signals are detected in blue and red domains in normal and abnormal spermatozoa respectively.
Shed Light on the Protein, Cell and Organelle Functions and Dynamics via Redox Biology, Such as:
- Correlate the cytochromes’ redox state with mitochondrial intermembrane potential and energy production
- Improve understanding of the link between mitochondrial dysfunction and neurodegenerative diseases or infertility
- Relate haemoglobin distribution in erythrocytes to their oxygen transport functions/abnormalities
- Study the oxidoreductase function of man-made redox proteins in synthetic biology
- Evaluate the oxygenation function of myoglobin in tissue engineering and in cancer progression
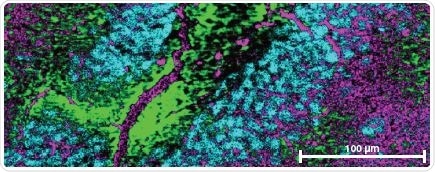
In tissue: analyze haem proteins’ distribution, level and redox state in situ – neurons (cyan) and glial cells (green and magenta) in the rat brain section displayed varied levels and redox states of cytochromes.
Raman Imaging: The Perfect Technique for Biological Research
- Compare the haem proteins’ electronic and physical properties between samples
- Simultaneously offer chemical information on other biomolecules, example, lipids, proteins, nucleic acids, minerals, carbohydrates
- Attain valuable insights of the effect of redox regulation on biological systems

The Renishaw inVia confocal Raman microscope.
Renishaw inVia: Perfect for Studying Redox Biology with Raman Spectroscopy
- Research grade confocal Raman microscope
- StreamLine™ imaging technology for high speed mapping of haem proteins without causing tissue/cell damage
- StreamLine imaging with Slalom for a rapid overview of tissue samples
- High confocality StreamHR™ imaging to scrutinize small details
Relevant Reading:
- Brazhe et al, 2012, Mapping of Redox State of Mitochondrial Cytochromes in Live Cardiomyocytes Using Raman Microspectroscopy, PLoS ONE 7(9): e41990
- Ramser et al, 2012, Resonance Micro-Raman Investigations of the Rat Medial Preoptic Nucleus: Effects of a Low-Iron Diet on the Neuroglobin Content, Applied Spectrosc 12:1454-60
- Brazhe et al, 2013, In Situ Raman Study of Redox State Changes of Mitochondrial Cytochromes in a Perfused Rat Heart, PLoS ONE 8(8): e70488
- Parshina et al, 2013, Combined Raman and atomic force microscopy study of hemoglobin distribution inside erythrocytes and nanoparticle localization on the erythrocyte surface, Laser Phys. Lett. 075607
- Anderson et al, 2014, Constructing a man-made c-type cytochrome maquette in vivo: electron transfer, oxygen transport and conversion to a photoactive light harvesting maquette, Chem. Sci. 5: 507-514
- Synytsya et al, 2014, Raman spectroscopy at different excitation wavelengths (1064, 785 and 532 nm) as a tool for diagnosis of colon cancer, J. Raman Spectrosc. 45(10): 903-911
This information has been sourced, reviewed and adapted from materials provided by Renishaw plc.
For more information on this source, please visit Renishaw plc.
Last updated: Feb 27, 2018 at 6:29 AM






















.png)












No hay comentarios:
Publicar un comentario