
Genetic Gymnastics Aid Our Sense of Touch
A Single Gene’s Varied Products Help Us Feel Our Complex World

Touch is a complicated sense that results from many different types of mechanical stimuli: Pressure, texture, vibrations, and more. Our sense of touch is sensitive enough to detect even the movement of a single hair. It allows us to appreciate the varied crunchy textures of carrots, the smoothness of cream, and “slipperiness” of noodles. Together with other senses, touch helps us decide what’s safe to eat. Pain-sensitive touch receptors in our teeth even alert us when it’s time to see a dentist.
“Sensing mechanical force—the sense of touch—is important for both pleasure and pain, and it contributes to our survival,” says Mark Hoon, PhD, a researcher at NIH’s National Institute of Dental and Craniofacial Research (NIDCR). “In the long run, a better understanding of touch might point to potential therapies for relieving pain or other unpleasant sensations.”
Recent research by Hoon and other NIH scientists has begun to unravel how touch detection is regulated at the molecular level to produce distinct responses in different classes of sensory cells. The study, published in Cell Reports, was led by NIDCR’s Hoon and Alexander Chesler, PhD, of NIH’s National Institute of Complementary and Integrative Health. The research establishes that many aspects of our sense of touch result from the genetic gymnastics of a single gene.

Surfaces within the mouth, around the face, and in other areas of the body are densely populated with nerve endings that sense mechanical changes and send signals via the spinal cord and brainstem onward to the brain. Different types of nerve cells transmit distinct types of sensory information, such as gentle touch, vibration, or proprioception, which is the orienting sense that allows you, for example, to touch your finger to your nose when your eyes are closed.
Previous studies have shown that one gene, called Piezo2, plays a critical role in all of these types of sensation. Piezo2 codes for a protein found predominantly, but not exclusively, in sensory neurons. The protein is found to a lesser extent in the lung, bladder, skin, and bone. Piezo2 resides in the cell membrane and acts as a gate for mechanical signals.
The researchers wondered how a single, large gene could process so many different types of sensory stimuli. They decided to focus on whether alternative splicing—a common genetic mechanism in which a single gene can produce different variants of the same protein—was the trick Piezo2 employed to create sensory diversity. Each of these variants can have distinct and specific functions in different types of cells.
Using mouse models and a recently developed gene-sequencing method, the researchers were able to record, sort, and compare the expression of the Piezo2 gene variants in tissue from lung, bladder, and trigeminal ganglion, a node of nerve cells next to the brainstem that receives sensory input from the head and face.
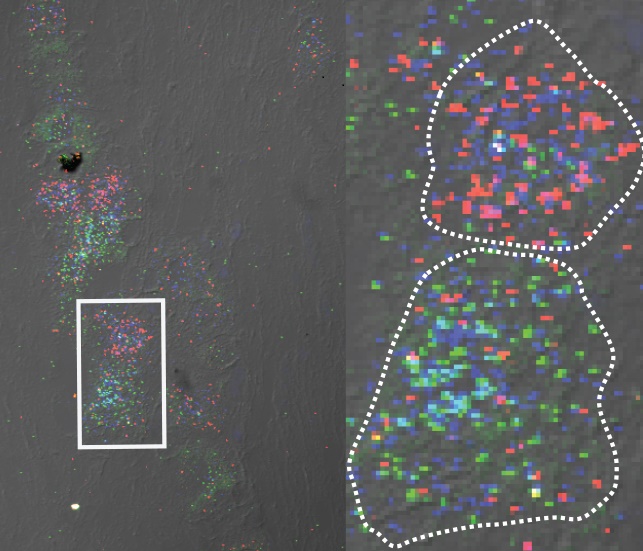
The study found that at the molecular level, Piezo2 is regulated differently in different types of tissues and even different types of cells. Sensory nerve cells have multiple forms of the Piezo2 protein, whereas other types of cells, such as lung and bladder cells, have only one predominant type. The team discovered that the protein variants behaved differently as well, suggesting this property enables certain types of nerve cells to detect specific types of sensation.
By examining Piezo2 expression in human sensory cells, the researchers confirmed that some of the mouse results also applied to humans. “The existence of multiple variants of a protein is a powerful way to generate functional complexity from a single gene,” Hoon says.
Next steps for the research team include looking at what happens not only in the lab, but also in living tissues. They will examine how different variants of the Piezo2 protein in mice determine the sensory function of the cell. Which variant proteins help us discriminate mushy from crunchy? Smooth from rough? Chewable from drinkable? The answers could have important implications for our understanding of touch and for future medical therapies related to touch, pain, and other sensations.
Next steps for the research team include looking at what happens not only in the lab, but also in living tissues. They will examine how different variants of the Piezo2 protein in mice determine the sensory function of the cell. Which variant proteins help us discriminate mushy from crunchy? Smooth from rough? Chewable from drinkable? The answers could have important implications for our understanding of touch and for future medical therapies related to touch, pain, and other sensations.
Reference
Marcin Szczot, Leah A. Pogorzala, Hans Jurgen Solinski, Lynn Young, Philina Yee, Claire E. Le Pichon, Alexander T. Chesler, and Mark A. Hoon. Cell-type-specific splicing of Piezo2 regulates mechanotransduction. Cell Reports. 2017;21(10):2760-2771.
NIH Support: This study was conducted by researchers at the National Institute of Dental and Craniofacial Research (NIDCR), National Institute of Complementary and Integrative Health (NCCIH), NIH Library, and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).





















.png)












No hay comentarios:
Publicar un comentario