
Volume 23, Number 8—August 2017
Research Letter
Human Infection with Burkholderia thailandensis, China, 2013
On This Page
Kai Chang, Jie Luo, Huan Xu, Min Li, Fengling Zhang, Jin Li, Dayong Gu, Shaoli Deng, Ming Chen , and Weiping Lu
, and Weiping Lu
Abstract
Burkholderia thailandensis infection in humans is uncommon. We describe a case of B. thailandensisinfection in a person in China, a location heretofore unknown for B. thailandensis. We identified the specific virulence factors of B. thailandensis, which may indicate a transition to a new virulent form.
Burkholderia thailandensis is closely related to B. pseudomallei, the causative agent of melioidosis (1). B. thailandensis shares most virulence factors and extensive genomic similarity with B. pseudomallei but can be distinguished by its ability to assimilate arabinose and different rRNA sequences (2,3). Little is known about B. thailandensis infection in humans. Two case reports described soft tissue infection and pneumonia with sepsis in Thailand and the United States (4,5). We describe a clinical investigation of human infection with B. thailandensisin Chongqing, China.
In October 2013, a 67-year-old man in Chongqing was hospitalized with a 13-day history of fever, productive cough with white sputum, and shortness of breath. Symptoms had not improved after antimicrobial drug treatment at a local clinic. The patient denied contact with any sick persons and any environmental exposure. Empirical treatment with meropenem was used to prompt resolution of the patient’s symptoms before culture results were received. During the 6-day treatment course, the patient was transferred to Chongqing Infectious Disease Hospital for treatment. Subsequently, his general condition worsened, and his family wished to have him close to home. He was discharged and died 2 days later.
Laboratory evaluations of blood samples performed at the time of the patient’s admission showed a leukocyte count of 20.72 × 109 cells/L with a markedly elevated 91.5% neutrophils, aspartate aminotransferase level of 75.5 U/L (reference range 15.0–40.0 U/L), alanine aminotransferase level of 85.0 U/L (reference range 9.0–50.0 U/L), interleukin-6 level of 352.1 pg/mL (reference range 0–7 pg/mL), and procalcitonin level of 24.37 ng/mL (reference range 0–0.25 ng/mL). A computed tomography scan of the patient’s chest showed a thick-walled cavitary lesion at the posterior segment of the right upper lobe measuring 7.9 × 6.1 cm and multiple nodules in both lung fields (Technical Appendix[PDF - 333 KB - 3 pages] Figure 1).
On day 6 of the patient’s hospitalization, we observed via microscopy that the positive blood culture contained many gram-negative rod-shaped bacteria (Technical Appendix[PDF - 333 KB - 3 pages] Figure 2, panel A). The colonies were smooth and glossy, with silver pigmentation, on sheep blood agar Technical Appendix[PDF - 333 KB - 3 pages] Figure 2, panel B). The VITEK 2 COMPACT system (bioMérieux, Marcy L’Étoile, France) identified the isolated strain as B. pseudomallei (97% probability; bionumber 0003451513500211). The API 20NE system (bioMérieux) also identified the isolated strain as B. pseudomallei (50.5% probability; index 1157577). However, the biochemical profiles of the API 20NE system, including arabinose assimilation, identified the isolated strain as B. thailandensis, based on the mode of artificial interpretation. We analyzed the 16S rDNA sequence of strain BPM with nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and found a 100% similarity with B. thailandensis (GenBank accession nos. CP000085.1 and CP000086.1).
These results indicate that commercially available phenotypic assays are not ideal for the identification of B. thailandensis, which has not yet been incorporated into the databases of identification systems (6,7). Moreover, the arabinose assimilation proved to be an effective, simple, and accurate method for differentiating B. thailandensis from B. pseudomallei. When B. pseudomallei is presumptively identified, arabinose assimilation should be emphasized in clinical laboratories.
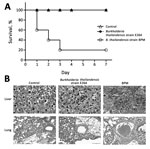
Figure. Virulence comparison of Burkholderia thailandensis isolated from a man in Chongqing, China, compared with B. thailandensis E264 (strain ATCC 700388). A) Survival pattern of 5 BALB/c mice intraperitoneally challenged with 107...
We compared the virulence of the isolated strain with B. thailandensis E264 (strain ATCC 700388) in BALB/c mice. B. thailandensis E264 is an environmental isolate from northeast Thailand. The clinically isolated B. thailandensis from this study was defined as strain BPM. Groups of 5 mice were inoculated with 107 CFU of each isolate and observed for a period of 7 days after infection. Four fifths of the mice infected with strain BPM died within 1 week of challenge. B. thailandensis could be isolated from the bloodstream of mice at the time of death. In contrast, all mice with B. thailandensis E264 infection survived over a 1-week monitoring period (Figure, panel A). The histologic findings were notable for early dissemination to the liver and lung (Figure, panel B). We observed multiple large, necrotizing foci in the livers of mice infected with strain BPM and alveolar-based neutrophilic inflammation in the strain BPM infection group. In addition, the inflammatory infiltrate and lung hyperemia were raised in the BPM-infected mice. This finding is consistent with the clinical case in our study, which appeared as pneumonia and sepsis. Overall, these experiments confirm that strain BPM is a virulent pathogen.
We performed comparative genomics to reveal the pathogenic mechanism of strain BPM. The BPM strain and B. thailandensis species share a large proportion of virulence factors. When compared with the reference genome sequences of B. thailandensis E264, B. thailandensis 2002721723, and B. thailandensis E444, the specific virulence factors of VirB/VirD4 type IV secretion system, HSI-I, and WcbR were indicated in strain BPM (Technical Appendix[PDF - 333 KB - 3 pages]Table) (8–10). These specific virulence factors may represent a transition toward a new virulent form.
In conclusion, when considering B. pseudomallei infection, clinicians should also consider the possibility of B. thailandensis infection. B. thailandensis is not identified with use of commercially available phenotypic assays and may be mistaken for B. pseudomallei. In the future, deep analysis of the complete genome would be helpful in understanding the evolution of B. thailandensis and its adaptation to the environment.
Dr. Chang is a medical doctor at the Third Military Medical University in Chongqing, China. His main research interests are medical microbiology and molecular diagnostics.
Acknowledgment
This study was supported by the National Natural Sciences Foundation of China (grant nos. 81401751, 81171667), Shenzhen Science and Technology Research and Development Fund (grant no. CXZZ20150504163004339), and the Guangdong Science and Technology Project (grant no. 20160223).
References
- Ngamdee W, Tandhavanant S, Wikraiphat C, Reamtong O, Wuthiekanun V, Salje J, et al. Competition between Burkholderia pseudomallei and B. thailandensis. BMC Microbiol. 2015;15:56. DOIPubMed
- Yu Y, Kim HS, Chua HH, Lin CH, Sim SH, Lin D, et al. Genomic patterns of pathogen evolution revealed by comparison of Burkholderia pseudomallei, the causative agent of melioidosis, to avirulent Burkholderia thailandensis. BMC Microbiol. 2006;6:46. DOIPubMed
- Haraga A, West TE, Brittnacher MJ, Skerrett SJ, Miller SI. Burkholderia thailandensis as a model system for the study of the virulence-associated type III secretion system of Burkholderia pseudomallei. Infect Immun. 2008;76:5402–11. DOIPubMed
- Lertpatanasuwan N, Sermsri K, Petkaseam A, Trakulsomboon S, Thamlikitkul V, Suputtamongkol Y. Arabinose-positive Burkholderia pseudomalleiinfection in humans: case report. Clin Infect Dis. 1999;28:927–8. DOIPubMed
- Glass MB, Gee JE, Steigerwalt AG, Cavuoti D, Barton T, Hardy RD, et al. Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J Clin Microbiol. 2006;44:4601–4. DOIPubMed
- Amornchai P, Chierakul W, Wuthiekanun V, Mahakhunkijcharoen Y, Phetsouvanh R, Currie BJ, et al. Accuracy of Burkholderia pseudomalleiidentification using the API 20NE system and a latex agglutination test. J Clin Microbiol. 2007;45:3774–6. DOIPubMed
- Zong Z, Wang X, Deng Y, Zhou T. Misidentification of Burkholderia pseudomallei as Burkholderia cepacia by the VITEK 2 system. J Med Microbiol. 2012;61:1483–4. DOIPubMed
- Schröder G, Schuelein R, Quebatte M, Dehio C. Conjugative DNA transfer into human cells by the VirB/VirD4 type IV secretion system of the bacterial pathogen Bartonella henselae. Proc Natl Acad Sci U S A. 2011;108:14643–8. DOIPubMed
- Yu Y, Yang H, Li J, Zhang P, Wu B, Zhu B, et al. Putative type VI secretion systems of Vibrio parahaemolyticus contribute to adhesion to cultured cell monolayers. Arch Microbiol. 2012;194:827–35. DOIPubMed
- Price EP, Sarovich DS, Mayo M, Tuanyok A, Drees KP, Kaestli M, et al. Within-host evolution of Burkholderia pseudomallei over a twelve-year chronic carriage infection. MBio. 2013;4:e00388-13. DOIPubMed






















.png)











No hay comentarios:
Publicar un comentario