A new DRUG TRIALS SNAPSHOT is now available.

REBLOZYL is a drug for the treatment of anemia in adults with beta thalassemia who require regular red blood cell transfusions.
Beta thalassemia is an inherited disorder of hemoglobin leading to anemia and other medical problems. Some patients may have severe anemia and need regular blood transfusions.
REBLOZYL is an injection given under the skin (subcutaneously) every three weeks.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: REBLOZYL
REBLOZYL ( luspatercept-aamt)
REB-loh-zil
Celgene Corporation
Approval date: November 8, 2019
REB-loh-zil
Celgene Corporation
Approval date: November 8, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
REBLOZYL is a drug for the treatment of anemia in adults with beta thalassemia who require regular red blood cell transfusions.
Beta thalassemia is an inherited disorder of hemoglobin leading to anemia and other medical problems. Some patients may have severe anemia and need regular blood transfusions.
How is this drug used?
REBLOZYL is an injection given under the skin (subcutaneously) every three weeks.
What are the benefits of this drug?
REBLOZYL was better than placebo in decreasing the need for red blood cell transfusions. In the trial 21% of patients receiving REBLOZYL achieved a 33% reduction in RBC transfusion in comparison to 5% of patients who received placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
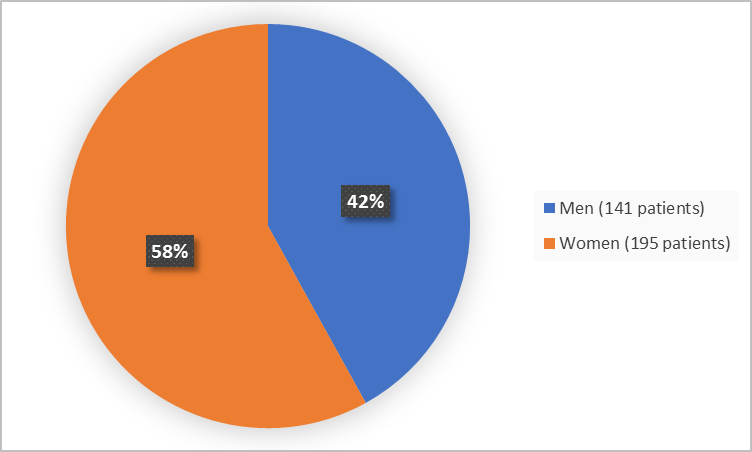
- Sex: REBLOZYL worked similarly in men and women.
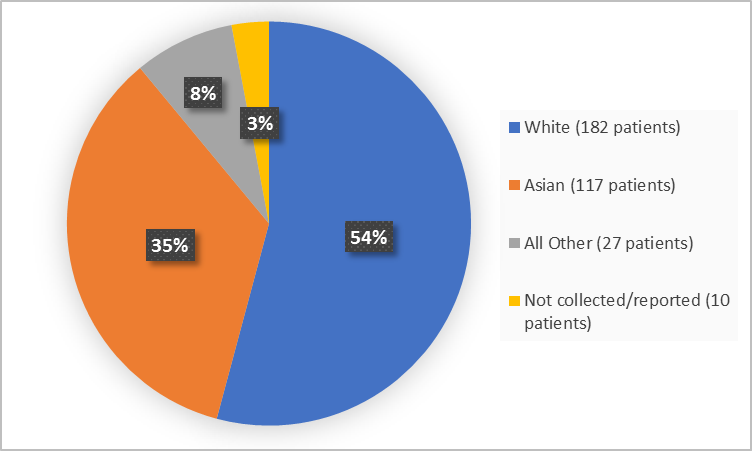
- Race: REBLOZYL worked imilarly in all tested race groups.
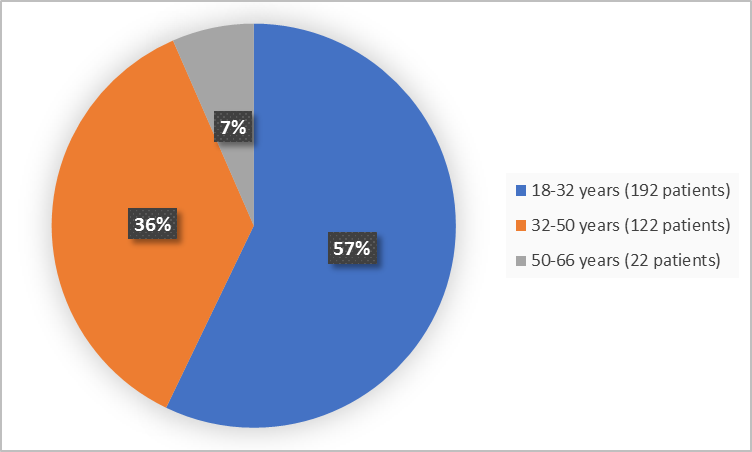
- Age: REBLOZYL worked similarly in all tested age groups. There were not enough patients older than 65 years to determine whether there was any difference between older and younger patients.
What are the possible side effects?
REBLOZYL can cause serious side effects including increased risk for blood clots and high blood pressure. REBLOZYL may cause harm to unborn babies.
The most common side effects of REBLOZYL are headache, bone pain and joint arthralgia, fatigue, cough, abdominal pain, diarrhea, and dizziness.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in all tested race groups.
- Age: The occurrence of side effects was similar in all tested age groups. There were not enough patients older than 65 years to determine whether there was any difference between older and younger patients.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved REBLOZYL based primarily on evidence from one clinical trial (Trial 1/ NCT02604433) of 336 adult patients 18-66 years old with beta thalassemia. The trial was conducted at 65 sites in 15 countries (Australia, Malaysia, Taiwan, Thailand, Israel, Lebanon, Tunisia, Turkey, Bulgaria, Canada, France, Greece, Italy, the United Kingdom, and the United States).
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Demographics by Race
FDA Review
Table 1. Demographics by Race
Race | Number of Patients | Percentage |
White | 182 | 54 |
Black or African American | 1 | Less than 1 |
Asian | 117 | 35 |
Other | 26 | 8 |
Not collected or not reported | 10 | 3 |
FDA Review
Figure 3 summarizes the percentage of patients by age group in the clinical trial.
Figure 3. Demographics by Age
Clinical Trial Data
How were the trials designed?
REBLOZYL was evaluated in one clinical trial of 336 adult patients with beta thalassemia who required regular red blood cell transfusions (6-20 red blood cell units per 24 weeks).
In the trial, patients were randomly assigned to receive either REBLOZYL or a matched placebo every three weeks subcutaneously as long as they required less transfusions or unacceptable toxicity occurred. Neither the patients nor the investigators knew which treatment was given until the end of the trial.
The benefit of REBLOZYL was assessed by proportion of patients whose need for transfusion was reduced at least 33% and comparing it to the proportion of placebo- treated patients who also achieved at least 33% transfusion reduction.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.


































No hay comentarios:
Publicar un comentario