A new DRUG TRIALS SNAPSHOT is now available.

BRUKINSA is used to treat adults with mantle cell lymphoma (MCL) who have received at least one prior treatment for their cancer.
MCL is a rare, rapidly progressing cancer that forms in the lymph system. MCL is one type of B-cell non-Hodgkin lymphoma.
BRUKINSA is a capsule. It may be taken as two capsules twice a day, or four capsules once a day.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots BRUKINSA
BRUKINSA (zanubrutinib)
BROO-kin-sah
BeiGene
Approval date: November 14, 2019
BROO-kin-sah
BeiGene
Approval date: November 14, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BRUKINSA is used to treat adults with mantle cell lymphoma (MCL) who have received at least one prior treatment for their cancer.
MCL is a rare, rapidly progressing cancer that forms in the lymph system. MCL is one type of B-cell non-Hodgkin lymphoma.
How is this drug used?
BRUKINSA is a capsule. It may be taken as two capsules twice a day, or four capsules once a day.
What are the benefits of this drug?
In the trials, about 84 percent of patients had a complete or partial shrinkage of their tumors after treatment.
BRUKINSA was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
More trials are ongoing to assess whether there is a clinical benefit.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: BRUKINSA worked similarly in men and women.
- Race: Most of the patients were Asians. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
- Age: BRUKINSA worked similarly in patients younger and older than 65 years of age.
What are the possible side effects?
BRUKINSA may cause serious side effects including bleeding, infections, decreased blood cell counts, new cancers and heart rhythm problems.
The most common side effects of BRUKINSA are low blood cell counts, upper respiratory tract infections, rash, bruising, diarrhea and cough.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: Most of the patients were Asians. Differences in the occurrence of side effects among races could not be determined because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved BRUKINSA based on evidence from two clinical trials (Trial 1/ NCT03206970 and Trial 2/ NCT 02343120) that evaluated 118 patients with MCL who had received at least one prior therapy.
Trial 1 was conducted at 13 sites in China, and Trial 2 was conducted at 25 sites in the United States, United Kingdom, Australia, New Zealand, Italy, and South Korea.
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Baseline Demographics by Race
Race | Number of Patients | Percentage |
White | 25 | 21 |
Asian | 89 | 76 |
Black or African American | 1 | Less than 1 |
Other | 3 | 3 |
FDA Review
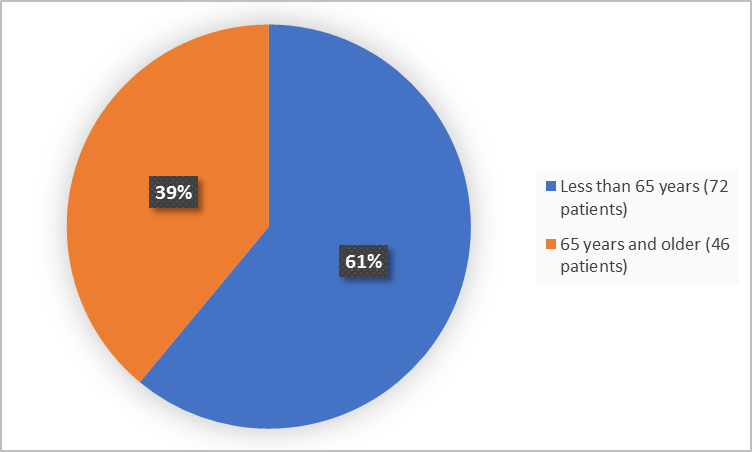
Figure 3 summarizes how many patients of certain age were in the clinical trials.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
There were two trials that evaluated the benefit and side effects of BRUKINSA.
Trial 1 enrolled patients with MCL who had received at least one prior therapy. All patients received two BRUKINSA capsules twice daily until disease progression or unacceptable side effects.
Trial 2 enrolled patients with B cell lymphoma including MCL patients who were previously treated with BRUKINSA. Patients received either two BRUKINSA capsules twice daily or three BRUKINSA capsules once a day until disease progression or unacceptable side effects.
The benefit of BRUKINSA was evaluated by measuring how many patients experienced complete or partial shrinkage of their tumors after treatment (overall response rate). In trial 1 patients underwent PET (positron emission tomography) imaging for tumor assessment, while in Trial 2, computed tomography or magnetic resonance imaging was used.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.




































No hay comentarios:
Publicar un comentario