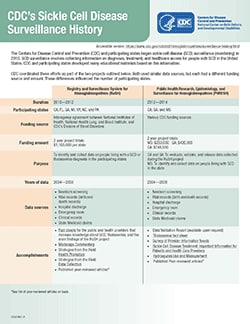
CDC’s Sickle Cell Disease Surveillance History
The Centers for Disease Control and Prevention (CDC) and participating states began sickle cell disease (SCD) surveillance (monitoring) in 2010. SCD surveillance involves collecting information on diagnoses, treatment, and healthcare access for people with SCD in the United States. CDC and participating states developed many educational materials based on this information.
CDC coordinated these efforts as part of the three projects outlined below. All three used similar data sources, but each had a different funding source and amount. These differences influenced the number of participating states.
| Registry and Surveillance System for Hemoglobinopathies (RuSH) | Public Health Research, Epidemiology, and Surveillance for Hemoglobinopathies (PHRESH) | Sickle Cell Data Collection (SCDC) Program | |
|---|---|---|---|
| Duration | 2010—2012 | 2012—2014 | Ongoing since 2015 |
| Participating states | CA, FL, GA, MI, NY, NC, and PA | CA, GA, and MS | CA (since 2015) and GA (since 2016) |
| Funding source | Interagency agreement between National Institutes of Health, National Heart, Lung, and Blood Institute, and CDC’s Division of Blood Disorders | Various CDC funding sources | CDC (Association of University Centers on Disabilities) and CDC Foundation (Pfizer, Bioverativ, Global Blood Therapeutics) |
| Funding amount | 2 year project totals: $1,100,000 per state | 2 year project totals: MS: $250,000 GA: $420,000 CA: $748,000 | Annual totals: CA: $400,000 GA: $123,600 |
| Purpose | To identify and collect data on people living with SCD or thalassemia in the participating states | CA and GA: To evaluate and validate data collected during RuSH and to share findings from the project MS: To identify and collect data on people living with SCD in the state | To study trends in diagnosis,treatment, and healthcare access and to share findings with audiences who will drive policy and healthcare changes that improve the lives of people with SCD |
| Years of data | 2004—2008 | 2004—2008 | 2004—2016 (data after 2016 will be collected as it becomes available) |
| Data sources |
|
|
|
| Accomplishments |
|
|
|
*See list of peer-reviewed articles below.

Published peer-reviewed articles
RuSH
- Wang Y, Kennedy J, Caggana M, Zimmerman R, Thomas S, Berninger J, Harris K, Green NS, Oyeku S, Hulihan M, Grant AM, Grosse SD. Sickle cell disease incidence among newborns in New York State by maternal race/ethnicity and nativity. Genet Med. 2013 Mar;15(3):222–8.
- Paulukonis ST, Harris WT, Coates TD, Neumayr L, Treadwell M, Vichinsky E, Feuchtbaum LB. Population based surveillance in sickle cell disease: methods, findings and implications from the California registry and surveillance system in hemoglobinopathies project (RuSH). Pediatr Blood Cancer. 2014 Dec;61(12):2271–6.
- Hulihan MM, Feuchtbaum L, Jordan L, Kirby RS, Snyder A, Young W, Greene Y, Telfair J, Wang Y, Cramer W, Werner EM, Kenney K, Creary M, Grant AM. State-based surveillance for selected hemoglobinopathies. Genet Med. 2015 Feb;17(2):125–30.
- Wang Y, Liu G, Caggana M, Kennedy J, Zimmerman R, Oyeku SO, Werner EM, Grant AM, Green NS, Grosse SD. Mortality of New York children with sickle cell disease identified through newborn screening. Genet Med. 2015 Jun;17(6):452–9.
PHRESH
- Neunert CE, Gibson RW, Lane PA, Verma-Bhatnagar P, Barry V, Zhou M, Snyder A. Determining Adherence to Quality Indicators in Sickle Cell Anemia Using Multiple Data Sources. Am J Prev Med. 2016 Jul;51(1 Suppl1):S24–30.
Additional Resources





















.png)












No hay comentarios:
Publicar un comentario