
Pain Among Cancer Survivors
ORIGINAL RESEARCH — Volume 17 — July 9, 2020
M. Shayne Gallaway, PhD, MPH1; Julie S. Townsend, MS1; Daniel Shelby, BS2; Mary C. Puckett, PhD1 (View author affiliations)
Suggested citation for this article: Gallaway MS, Townsend JS, Shelby D, Puckett MC. Pain Among Cancer Survivors. Prev Chronic Dis 2020;17:190367. DOI: http://dx.doi.org/10.5888/pcd17.190367.
PEER REVIEWED
On This Page
Summary
What is already known about this topic?
Pain is one of the most common symptoms that cancer survivors experience.
What is added by this report?
We investigated the demographic and physiologic characteristics of cancer survivors who experience pain.
What are the implications for public health practice?
Knowledge of the demographic and physiologic characteristics of cancer survivors most likely to report experiencing cancer or cancer treatment–related pain can help educate clinicians, cancer survivors, and caregivers and inform regular screening for and proper characterization of pain, pain treatment methods, and ongoing monitoring of treatment efficacy.
Abstract
Introduction
Pain is one of the most common symptoms that people with cancer experience. Identification of demographic, physiologic, and behavioral correlates of pain among cancer survivors could help identify subgroups most in need of pain management.
Methods
We analyzed data from the 2012, 2014, and 2016 Behavioral Risk Factor Surveillance System Cancer Survivorship Optional Module, which was completed by 18 states and territories, to describe demographic and physiologic characteristics of cancer survivors reporting physical pain caused by cancer or cancer treatment. Adjusted and unadjusted population-based estimates and 95% confidence intervals were calculated.
Results
Of 12,019 cancer survivor respondents, 9.5% reported current pain related to cancer or cancer treatment. Current pain differed significantly by sex, race/ethnicity, age, and cancer type. Current pain was reported most often among survivors with more than 3 chronic diseases (16.7%) compared with survivors with none (8.1%) or 1 or 2 (10.0%). Pain was higher among survivors reporting fair or poor general health (18.0%) than among survivors reporting otherwise, and higher among survivors reporting more than 14 days of poor physical health (16.6%) or poor mental health (14.8%) compared with less than 14 days (in the past 30 days).
Conclusions
Our results suggest that approximately 10% of cancer survivors in the United States are experiencing pain that may have persisted for years after their initial diagnosis and may not be adequately controlled. Increasing knowledge of the most appropriate pain management planning and strategies for controlling short- and long-term chronic pain among cancer survivors could help reduce the prevalence of pain.
Introduction
Approximately 15.5 million cancer survivors (people who received a diagnosis of cancer) were alive in the United States in 2016, and that number is expected to increase to nearly 20 million by 2026 (1,2) because nearly half of cancer survivors live longer than 10 years (1). Pain is one of the most common symptoms experienced among cancer patients and can be caused by cancer itself (eg, tumor pressing on nerves, bones, or organs), surgery, treatment and treatment side effects (eg, peripheral neuropathy, mouth sores, radiation mucositis), or other procedures and tests (3,4). Research suggests that pain occurs in approximately 20% to 50% of cancer survivors (4,5). Clinical factors that may be associated with survivor pain are the stage (type and invasiveness) of the tumor, type of anticancer treatment received, time since completing treatment, comorbid conditions, and initial pain management (3,6–8). Effective methods are available to prevent and control pain during and after cancer treatment, including early recognition of pain symptoms, characterization and communication about pain type and severity, pharmacologic and nonpharmacologic pain control options, and patient education to ensure adequate pain and symptom management through all phases of cancer treatment and following treatment (9–11). Although pain can be controlled, approximately 30% of cancer survivors do not receive pain medication proportional to their pain intensity (12). Pain can negatively affect a cancer survivor’s daily functional status and quality of life (7,8,13) and can persist for years. Cancer survivors may experience psychological distress when pain persists after completion of cancer treatment (4), and untreated pain can lead to unnecessary hospital admissions (14,15). Identification of demographic, physiologic, and behavioral correlates of pain in cancer survivors can provide important information on specific subgroups most in need of pain management.
Cancer survivors can suffer from both short- and long-term pain (3); however, treatment-related pain typically diminishes over time. Approximately 1 year after diagnosis, more than 90% of patients observed in the American Cancer Society’s Study of Cancer Survivors-I study reported short-term pain symptoms related to their cancer or its treatment (7); 6% of Australian adult cancer survivors reported pain intensity as “quite a bit/very much” 5 to 6 years post-diagnosis (8), and approximately 20% of childhood cancers survivors (with a mean survival time from diagnosis of 16.5 years) reported recent pain attributed to their previous cancer or cancer treatment (6). Pain may be more common among certain subpopulations, such as breast and lung cancer survivors, because of the cancer stage or surgery received (14,16). The prevalence and severity of chronic pain among cancer survivors has also been shown to vary by racial populations (ie, pain severity reported among blacks is greater than among whites) and sex (ie, occurrence of pain reported among females is greater than among males) (13).
Pain can also be associated with other physiologic symptoms (13,17). Cancer survivors who report pain also report lack of sleep, fatigue, and mental health issues (13,17). Patients with comorbid conditions may have significantly greater physical functional pain and associated limitations and may be less likely to improve with standard pain management (9).
Despite all the evidence related to the prevalence of pain and comorbidities with pain and the availability of effective pain management strategies, cancer survivors may not be fully aware of the long-term prevalence of cancer-related pain that may persist after treatment completion. Thus, behaviors associated with pain need to be better characterized to help inform clinicians treating cancer survivor populations that could most benefit from additional education, resources, and strategies to manage cancer-related chronic pain. To this end, the purpose of our study was to use the most current national data to describe demographic and physiologic characteristics of cancer survivors who reported physical pain caused by cancer or cancer treatment. Informing patients and providers will aid in promoting collaborative relationships critical to providing optimal pain management.
Methods
Survey
We analyzed cross-sectional data from the 2012, 2014, and 2016 Behavioral Risk Factor Surveillance System (BRFSS), a representative, state-based telephone survey that recruits residents via landline or cellular telephone (18). We used data from states and territories that administered the Cancer Survivorship Optional Module (Alabama, Alaska, Georgia, Hawaii, Idaho, Iowa, Indiana, Kansas, Louisiana, Michigan, Mississippi, Missouri, Nebraska, Ohio, South Dakota, Virgin Islands, Vermont, and Wisconsin). The median response rates by year among states with the survivorship module were 50.1% (2012), 50.2% (2014), and 51.4% (2016) and were representative of the populations surveyed (18). Respondents were asked if they had ever been told by a doctor, nurse, or other health care professional that they had cancer. If they answered yes, they were asked which type or how many different types of cancer they had and their age at first diagnosis. We excluded cancer survivors who reported nonmelanoma skin cancer, were unsure of their cancer type, or refused to provide a response to these questions.
Pain and demographic characteristics
We compared demographic and physiologic characteristics among all cancer survivors who did and did not report cancer pain or cancer treatment–related pain. To assess cancer pain and cancer treatment–related pain, respondents were asked, “Do you currently have physical pain caused by your cancer or cancer treatment?” Those who answered yes were classified as experiencing physical pain, regardless of when their most recent cancer diagnosis was. They were also asked, “Is your pain currently under control?” Demographics assessed were sex; age (18–39, 40–49, 50–65, ≥65 ); race/ethnicity (non-Hispanic white, non-Hispanic black, other (Asian, American Indian, Alaskan Native, Pacific Islander, other, Hispanic [of any race]); education (less than high school diploma, high school diploma or general education diploma (GED), some college [no degree or an associate degree], college degree [undergraduate degree, graduate degree]); current health insurance (yes or no); currently employed (yes or no); and whether medical care costs have restricted care (yes or no). We calculated and categorized years since diagnosis by using the respondents’ current age and age at first cancer diagnosis (≤5, 6–10, >10).
Physiologic characteristics
Respondents were asked to describe their general health as either excellent, very good, good, fair, or poor. They were also asked how many days in the past month (past 30 days) their physical health and mental health were good or better and how many days they considered their general health as poor. They reported the following behaviors for the month preceding the survey: average number of hours of sleep per night (subsequently categorized as <7 h or ≥7 h), smoking status (yes or no), binge drinking (yes or no), and co-occurring chronic diseases (arthritis, asthma, chronic obstructive pulmonary disease, depression, diabetes, heart disease, heart attack, kidney problems, stroke). We compared the proportion of respondents experiencing physical pain who described their general health as fair or poor (vs good, very good, or excellent) and also who reported 14 or more days (vs fewer than 14 days) of poor general health, mental health, or physical health.
Statistical analysis
We used SAS/SUDAAN version 11.0.1 (Research Triangle Institute) statistical software for analyses to account for the complex BRFSS design. Kansas, Nebraska, and Ohio elected to administer the survivorship module on a subset of their sample, so their survey weights accounted for this split survey design. Survey weights were also adjusted for states included in multiple years (Missouri, Nebraska, and Wisconsin). All estimates were weighted to provide population-based estimates, and 95% confidence intervals (CIs) were generated; 95% CIs are presented to allow for informal comparisons among prevalence estimates, without specifying a referent group. To determine how pain affects health status, significant associations between pain and health status (general, physical, and mental), adjusted prevalence estimates (ie, predicted marginals) were generated from multivariable logistic regression models adjusted for demographic and clinical factors. Best-fit models were determined through backward deletion of least significant variables. Given there was no human subject contact during completion of these secondary data analyses, institutional review board approval was not required.
Results
Of the 12,019 cancer survivor respondents (total weighted sample N = 2,949,032) who participated in the BRFSS during the study period, 9.5% (n = 1,146) reported experiencing pain related to cancer or cancer treatment. Female cancer survivors (12.5%) reported more physical pain related to cancer treatment than males (8.9%), and non-Hispanic black cancer survivors (22.9%) reported more physical pain related to cancer treatment than non-Hispanic whites (10.0%) and other racial/ethnic populations (11.2%) (Table 1). Cancer survivors aged 65 or older (6.2%) reported less pain than survivors younger than 65 (13.6%–20.3%). Survivors who were out of work or unable to work were also more likely to report physical pain related to cancer treatment (23.9%) than cancer survivors who were employed (11.5%), retired (5.8%), or reported another employment status (13.8%). Physical pain related to cancer treatment did not differ substantially among cancer survivors without current health insurance (15.2%) and survivors with current health insurance (10.8%); however, cancer survivors who reported that medical costs restricted their health care were more likely to report current pain (21.6%) related to cancer treatment than those who did not report health care restrictions because of medical costs (9.8%).
Survivors of lung cancer (28.3%), female breast cancer (19.1%), leukemia/lymphoma (18.0%), and colorectal cancer (15.7%) reported more physical pain related to cancer treatment than survivors with other cancers in our study (Table 2). Cancer survivors who were aged 65 or older at diagnosis were less likely to report physical pain (5.9%) related to treatment than survivors diagnosed when they were younger than 65 (10.1%–15.3%). Cancer survivors whose cancer was diagnosed more than 10 years in the past (9.5%) and survivors whose cancer was diagnosed 10 years or less in the past (11.9%–13.0%) reported a similar prevalence of physical pain related to cancer treatment.
Cancer survivors who reported 3 or more other chronic diseases (16.7%) reported more physical pain than survivors with none (8.1%) or 1 or 2 (10.0%) other chronic diseases (Figure 1). Current smokers (15.5%) also reported more physical pain than current nonsmokers (10.0%), and cancer survivors who reported sleeping less than 7 hours nightly (15.0%) reported more physical pain than those who slept 7 hours or more nightly (9.1%).
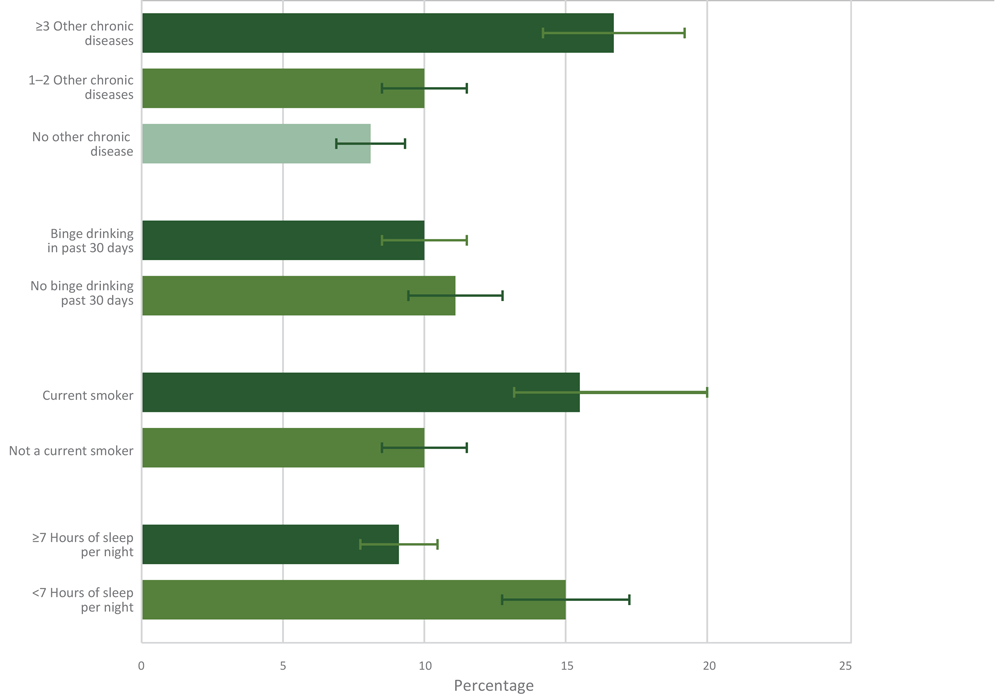
Figure 1.
Prevalence and 95% confidence intervals of current pain related to cancer or cancer treatment by comorbid and behavioral characteristics. Data on hours of sleep per night among cancer survivors from the Behavioral Risk Factor Surveillance System were available only for 2014 and 2016 (n = 9,910). Other chronic diseases included were a history of arthritis, asthma, chronic obstructive pulmonary disease, depression, diabetes, heart attack, heart disease, kidney disease, and stroke. Brackets indicate confidence intervals. [A tabular version of this figure is available.]
The unadjusted prevalence of physical pain was higher among cancer survivors who reported fair or poor general health (20.3%) than among survivors who reported their general health as good, very good, or excellent (7.3%) (Figure 2). Physical pain was higher among cancer survivors who reported 14 or more days of poor general health (22.8%), poor physical health (20.4%), or poor mental health (22.0%) compared with survivors who reported less than 14 days of each (13.1%, 8.6%, and 9.3%, respectively). The adjusted prevalence of physical pain was higher among cancer survivors who reported fair or poor general health (18.0%), 14 or more days of poor physical health (16.6%), or 14 or more days of poor mental health (14.8%) compared with survivors reporting excellent, very good, or good health, or less than 14 days of poor physical or mental health (in the past 30 days) (Table 3).
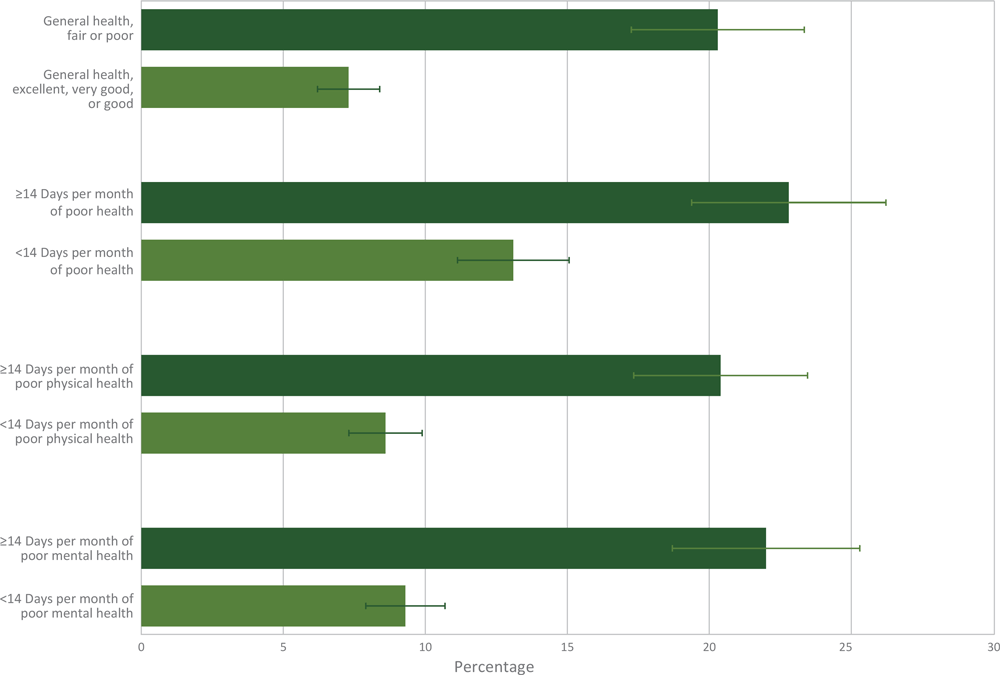
Figure 2.
Percentage and 95% confidence intervals of current pain related to cancer or cancer treatment by physical and mental health characteristics. Brackets indicate confidence intervals. [A tabular version of this figure is available.]
Discussion
Cancer survivors commonly experience pain in the months and years after a cancer diagnosis (3,6–8). As cancer evolves into a chronic illness, so, too, may chronic pain. The characteristics associated with increased pain have population health implications that may help patients and providers to initiate collaborative discussions of optimal pain management. Pain that persists can have a negative effect on daily functioning and other possible negative outcomes (9,13,17), and patients deserve safe and effective pain management. Our findings indicate that from 2012 through 2016, nearly 10% of cancer survivors reported experiencing pain related to cancer or cancer treatment, and approximately 20% experiencing pain reported that pain was inadequately controlled.
Pain can be caused by the cancer itself, and those experiencing more advanced cancer are more likely to have pain (4,19). Pain can also be caused by cancer-related treatment or tests (4). Pain has been shown to be greatest among patients with certain cancers (14). Consistent with previous studies, our study found cancer-related pain to be highest among survivors of lung cancer, female breast cancer, leukemia/lymphoma, and colorectal cancer. Breast cancer survivors may experience increased pain related to tumor size, location, lymphedema, and potential spread to the nervous system, which can cause lingering neuropathic pain (16,19). Lung cancer survivors may experience increased pain at any cancer stage of development (ie, early or advanced). Such pain is usually of mixed pathophysiology, and a relatively high proportion is often attributed to neurologic damage from cancer treatment or metastasis to other organs (20). However, pain may also be increased among other cancer types that we were unable to examine individually in our study. Comprehensive assessment, including the effect of pain on function and quality of life, is important for all survivors (11), and long-term assessment can help to better recognize novel or previously unrecognized painful consequences of treatment (11). Effective pain management can generally be accomplished by regular screening (to recognize pain early), proper characterization of the pain (eg, acute or chronic, secondary to cancer, breakthrough), determination of optimal pharmacologic or nonpharmacologic treatment options, proper education for patients, and patient follow-up over time to titrate and adjust treatments (21). Useful clinical practice guidelines on unique considerations for use of opioids for pain control in cancer survivors are provided in the 2016 American Society of Clinical Oncology Clinical Practice Guideline on Management of Chronic Pain in Survivors of Adult Cancers and the 2018 National Comprehensive Cancer Network Clinical Practice Guideline for Prescribing Opioids for Chronic Pain (10).
Cancer-related pain control could be addressed early after a cancer diagnosis because inadequate control can lead to subsequent hospital visits and interference with daily activities (9). In our study, approximately 1 in 5 cancer survivors who experienced pain reported that it was inadequately controlled. Survivors reported pain related to cancer and cancer treatment for many years after the initial diagnosis. Telephone or web-based supportive care (22) or patient navigation (23) may help mitigate inadequate pain control. Public health programs can use these findings of characteristics associated with increased pain among cancer survivors to develop and implement interventions aimed at cancer survivors. The Centers for Disease Control and Prevention (CDC) National Comprehensive Cancer Control Program (NCCCP) awardees (ie, more than 60 state, territory, and tribal organization grant recipients) work in communities across the nation to prevent cancer, promote healthy lifestyles, and enhance cancer survivors’ quality of life (24). NCCCP awardees can seek partnership opportunities with health professionals to ensure that evidence-based guidelines and effective therapies are being applied. Given that studies have documented a high prevalence of inadequate pain control (12), physicians could consider including both short- and long-term pain management as part of cancer survivorship care plans and comprehensive palliative care strategies. Educating patients about how to routinely document and estimate pain between appointments may increase understanding of expected pain symptoms (15).
We found that cancer survivors who experienced pain were more likely to report a lack of sleep, comorbid chronic diseases, and smoking. Sleep disorders and mental health issues are common conditions in people with chronic pain (13,17,25), present in 17% and 90% of all adults in general, respectively (9). Many chronic pain syndromes include sleep disturbances, mood alterations, fatigue, and neurocognitive changes, which decrease quality of life (13,17). Chronic pain results in poor health-related quality of life among those with physical and mental health disorders (25,26). Cancer survivors in our study who reported pain were significantly more likely to report more than 14 days per month of poor general, physical, and mental health, which may lead to functional limitations (9). Given our findings and those of others, patients with multiple chronic conditions may require additional pain management strategies.
Ethnic and socio-demographic disparities are important considerations in care related to the prevalence, treatment, progression, and outcomes of pain management. The incidence and severity of chronic pain among cancer survivors has been shown to vary between racial and ethnic populations and by sex (13). Pain type, pain severity, number of pain locations, perceived etiology, pain interference, disability, functioning, and pain symptoms were all disproportionately higher among black respondents than among white respondents and among female respondents than among male respondents (13). Our findings were consistent with pain being more commonly reported among black cancer survivors than among white survivors and others and among female survivors. We also found that older cancer survivors (≥65) and cancer survivors who were first diagnosed when they were aged 65 or older reported cancer-related pain less. The most common types of cancer diagnosed among persons 65 or older were cancers of the lung, colon and rectum, stomach, breast, and prostate (2), but why people diagnosed at an older age were less likely to report pain is not entirely understood. A previous study of breast cancer survivors similarly found that younger age was associated with higher risk for chronic pain (27). These findings need to be interpreted cautiously because blacks in general (28) and elderly cancer patients (29) are at higher risk of undertreatment of chronic pain. Perceived discrimination and hopelessness have been implicated as reasons for this disparity among blacks (28); and elderly cancer patients are at higher risk for undertreatment because of assessment barriers such as memory or hearing loss, confusion, fear of being a burden, and stoicism (29).
Our study is subject to at least 5 limitations. First, BRFSS data are self-reported and are subject to the limitations associated with these types of data collection instruments, including respondent recall and social desirability bias. In particular, measures in the cancer survivor module to capture pain and pain management (eg, medication use and adherence) were brief and not as comprehensive as typical diagnostic pain assessment instruments used in clinical settings. A multidimensional pain instrument to capture pain severity, type, and functional impact would be preferable to characterize and inform pain management for cancer survivors (21). However, numerous studies have examined issues related to the reliability and validity of BRFSS and the system’s ability to provide valid estimates. Second, BRFSS provides limited information regarding respondents’ disease status (eg, disease-free, recurrence, end-of-life); we were only able to identify survivors currently being treated for cancer. Third, the cross-sectional nature of the data used for this study does not allow us to determine temporality for the relationships between pain and the factors examined. Fourth, BRFSS data on cancer-related pain were limited to the 18 states and territories that included the cancer survivor module during the years examined; these data are representative of only those survivors who responded to the survey in these states. Finally, it is possible that bias was introduced into these data because cancer survivors with the highest amount of pain may have been less willing or unable to participate in the survey.
Adequate palliative care for pain and symptom management through all phases of cancer treatment is a major concern for cancer survivors (30). Cancer survivors may not be aware how cancer-related pain may negatively affect their health and functioning in the years following treatment (9); thus, survivor characteristics associated with increased pain could help inform health care providers of cancer survivor populations that could most benefit from additional information to manage cancer-related chronic pain. The CDC NCCCP supports increasing knowledge of the most appropriate pain management planning and strategies for addressing short- and long-term chronic pain among cancer survivors (10,11,30). Partnerships between NCCCP cancer coalitions, health care providers, and treatment facilities may increase assessment, management, and planning for chronic pain among cancer survivors. Clinicians could also consider educating cancer survivors on available resources and strategies (21) to enhance informed decision making and alleviate patient fears related to pain medications (30).
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention. All authors were involved in the conceptualization, methodology, formal analysis, investigation, writing, and editing of this article. The authors have no conflicts of interest. This work did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
Author Information
Corresponding Author: Shayne Gallaway, PhD, MPH, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Cancer Prevention and Control, 4770 Buford Highway, Atlanta, GA 30341. Telephone: 602-542-2911. Email: MGallaway@cdc.gov.
Author Affiliations: 1Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Cancer Prevention and Control, Atlanta, Georgia. 2Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Violence Prevention, Atlanta, Georgia.
References
- Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016;25(7):1029–36. CrossRef PubMed
- de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev 2013;22(4):561–70. CrossRef PubMed
- Levy MH, Chwistek M, Mehta RS. Management of chronic pain in cancer survivors. Cancer J 2008;14(6):401–9. CrossRef PubMed
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 2016;51(6):1070–1090.e9. CrossRef PubMed
- Jensen MP, Chang HY, Lai YH, Syrjala KL, Fann JR, Gralow JR. Pain in long-term breast cancer survivors: frequency, severity, and impact. Pain Med 2010;11(7):1099–106. CrossRef PubMed
- Lu Q, Krull KR, Leisenring W, Owen JE, Kawashima T, Tsao JCI, et al. Pain in long-term adult survivors of childhood cancers and their siblings: a report from the Childhood Cancer Survivor Study. Pain 2011;152(11):2616–24. CrossRef PubMed
- Smith T, Stein KD, Mehta CC, Kaw C, Kepner JL, Buskirk T, et al. The rationale, design, and implementation of the American Cancer Society’s studies of cancer survivors. Cancer 2007;109(1):1–12. CrossRef PubMed
- Zucca AC, Boyes AW, Linden W, Girgis A. All’s well that ends well? Quality of life and physical symptom clusters in long-term cancer survivors across cancer types. J Pain Symptom Manage 2012;43(4):720–31. CrossRef PubMed
- Chang KL, Fillingim R, Hurley RW, Schmidt S. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent 2015;432:21–6. PubMed
- Meghani SH, Vapiwala N. Bridging the critical divide in pain management guidelines from the CDC, NCCN, and ASCO for cancer survivors. JAMA Oncol 2018;4(10):1323–4. CrossRef PubMed
- Paice JA, Portenoy R, Lacchetti C, Campbell T, Cheville A, Citron M, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34(27):3325–45. CrossRef PubMed
- Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol 2014;32(36):4149–54. CrossRef PubMed
- Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer 2011;117(9):1994–2003. CrossRef PubMed
- Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol 2011;29(19):2683–8. CrossRef PubMed
- Scholer AJ, Mahmoud OM, Ghosh D, Schwartzman J, Farooq M, Cabrera J, et al. Improving cancer patient emergency room utilization: a New Jersey state assessment. Cancer Epidemiol 2017;51:15–22. CrossRef PubMed
- Forsythe LP, Alfano CM, George SM, McTiernan A, Baumgartner KB, Bernstein L, et al. Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat 2013;137(2):617–30. CrossRef PubMed
- Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol 2012;30(30):3687–96. CrossRef PubMed
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System (BRFSS). 2018; https://www.cdc.gov/brfss/index.html. Accessed February 9, 2019.
- Freynhagen R, Bennett MI. Diagnosis and management of neuropathic pain. BMJ 2009;339:b3002. CrossRef PubMed
- Potter J, Higginson IJ. Pain experienced by lung cancer patients: a review of prevalence, causes and pathophysiology. Lung Cancer 2004;43(3):247–57. CrossRef PubMed
- National Cancer Institute. Cancer Pain (PDQ®)–Health Professional Version. 2019; https://www.cancer.gov/about-cancer/treatment/side-effects/pain/pain-hp-pdq. Accessed March 14, 2019.
- Kondo S, Shiba S, Udagawa R, Ryushima Y, Yano M, Uehara T, et al. Assessment of adverse events via a telephone consultation service for cancer patients receiving ambulatory chemotherapy. BMC Res Notes 2015;8(1):315. CrossRef PubMed
- Enard KR, Ganelin DM. Reducing preventable emergency department utilization and costs by using community health workers as patient navigators. J Healthc Manag 2013;58(6):412–27, discussion 428. CrossRef PubMed
- Centers for Disease Control and Prevention. National Comprehensive Cancer Control Program (NCCCP). 2019; https://www.cdc.gov/cancer/ncccp/index.htm. Accessed December 18, 2018.
- Dominick CH, Blyth FM, Nicholas MK. Unpacking the burden: understanding the relationships between chronic pain and comorbidity in the general population. Pain 2012;153(2):293–304. CrossRef PubMed
- Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med 2008;70(8):890–7. CrossRef PubMed
- Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009;302(18):1985–92. CrossRef PubMed
- Ezenwa MO, Fleming MF. Racial disparities in pain management in primary care. J Health Dispar Res Pract 2012;5(3):12–26. CrossRef PubMed
- Moye J, June A, Martin LA, Gosian J, Herman LI, Naik AD. Pain is prevalent and persisting in cancer survivors: differential factors across age groups. J Geriatr Oncol 2014;5(2):190–6. CrossRef PubMed
- Centers for Disease Control and Prevention, Lance Armstrong Foundation. A national action plan for cancer survivorship: advancing public health strategies. 2003; https://www.cdc.gov/cancer/survivors/pdf/plan.pdf. Accessed January 3, 2019.





















.png)












No hay comentarios:
Publicar un comentario