Volume 26, Number 7—July 2020
Dispatch
Severe Acute Respiratory Syndrome Coronavirus 2 Infection among Returnees to Japan from Wuhan, China, 2020
On This Page
Figures
Tables
Downloads
Article Metrics
Yuzo Arima , Tomoe Shimada, Motoi Suzuki, Tadaki Suzuki, Yusuke Kobayashi, Yuuki Tsuchihashi, Haruna Nakamura, Kaoru Matsumoto, Asuka Takeda, Keisuke Kadokura, Tetsuro Sato, Yuichiro Yahata, Noriko Nakajima, Minoru Tobiume, Ikuyo Takayama, Tsutomu Kageyama, Shinji Saito, Naganori Nao, Tamano Matsui, Tomimasa Sunagawa, Hideki Hasegawa, Makoto Ohnishi, and Takaji Wakita
, Tomoe Shimada, Motoi Suzuki, Tadaki Suzuki, Yusuke Kobayashi, Yuuki Tsuchihashi, Haruna Nakamura, Kaoru Matsumoto, Asuka Takeda, Keisuke Kadokura, Tetsuro Sato, Yuichiro Yahata, Noriko Nakajima, Minoru Tobiume, Ikuyo Takayama, Tsutomu Kageyama, Shinji Saito, Naganori Nao, Tamano Matsui, Tomimasa Sunagawa, Hideki Hasegawa, Makoto Ohnishi, and Takaji Wakita
Abstract
In early 2020, Japan repatriated 566 nationals from China. Universal laboratory testing and 14-day monitoring of returnees detected 12 cases of severe acute respiratory syndrome coronavirus 2 infection; initial screening results were negative for 5. Common outcomes were remaining asymptomatic (n = 4) and pneumonia (n = 6). Overall, screening performed poorly.
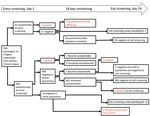
With the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, several countries, including Japan, repatriated their nationals (1–3). During January 29–31, 2020, a total of 566 Japanese nationals were repatriated via 3 chartered flights from Wuhan (206, 210, and 150 passengers). After passengers disembarked in Tokyo, Japan, quarantine officials assessed them for signs/symptoms (e.g., fever, respiratory illness) of coronavirus disease (COVID-19). A total of 28 symptomatic passengers were transferred to select hospitals for isolation. The remaining 538 were transported to a designated hospital, where another 35 were found to be symptomatic and were hospitalized there or transferred to other hospitals, leaving 503 asymptomatic persons for observation in quarantine (Figure).
We conducted day-1 entry screening by collecting oropharyngeal swab samples from all 566 returnees at the hospitals to which they were initially transported and testing them for SARS-CoV-2; all tests were based on the real-time reverse transcription PCR developed by the National Institute of Infectious Diseases (4). Hospitalized patients in isolation and asymptomatic returnees in quarantine were monitored daily for 14 days. If any signs/symptoms developed in a quarantined person, that person was transported to a designated hospital and oropharyngeal swab samples were collected for testing. We conducted exit screening for quarantined persons who remained illness-free by collecting oropharyngeal swab samples on day 14. The National Institute of Infectious Diseases Ethics Committee approved the study (registration no. 1096), and all 566 returnees who provided specimens gave written informed consent.
Among the 63 passengers who were symptomatic at entry screening, 2 (3.2%) were positive by PCR (Figure); test results were subsequently positive for 2 more. For 1 of these patients, pneumonia was diagnosed on day 1 and a sputum sample was positive on day 3; the other patient had fever and cough on day 1, pneumonia diagnosed on day 2, and a positive oropharyngeal swab sample on day 6. Excluding 1 patient who remained hospitalized for stroke, the remaining 58 patients were transferred to designated quarantine facilities after confirmation of good health and negative PCR results; all 58 remained asymptomatic after discharge, and PCR results were negative at exit screening.
For the 503 asymptomatic/subclinical passengers, entry-screening PCR results were positive for 5 (1.0%) (Figure); 3 remained asymptomatic, but mild signs/symptoms (fever, headache, sore throat) developed for 2 persons (1 on day 2, 1 on day 4). Of the remaining 498 persons with negative PCR results, 484 were quarantined at designated facilities and 14 at home. During quarantine, fever developed in 1 facility-quarantined and 1 home-quarantined person on day 10; both were confirmed positive by PCR, and pneumonia subsequently developed in both. The facility-quarantined case-patient was in a single room; no other person from this facility acquired COVID-19 or had a positive test result at exit screening. One person who remained asymptomatic had a positive test result at exit screening. Exit-screening results are pending for the patient hospitalized for stroke and the remaining 13 home-quarantined persons.
Among the 566 returnees, 12 cases of SARS-CoV-2 infection were detected; 540/541 facility-quarantined persons were confirmed negative by PCR performed on days 1 and 14 (197/197, 199/199, and 144/145 for the 3 flights). Entry screening detected 7 infections, for an infection point prevalence of 1.2%; infection period prevalence was 2.2% (12/552 returnees with complete follow-up). Despite universal testing, entry screening captured only 7/12 cases (58.3% sensitivity). Although screening symptomatic passengers (3.2%) was more efficient than screening all passengers (1.0%), screening only symptomatic passengers missed 5/7 prevalent infections at entry. Among symptomatic passengers, with 2 initially negative persons subsequently testing positive, entry-screening sensitivity was 2/4 (50%). Among asymptomatic passengers, with 3 initially negative persons subsequently testing positive, entry-screening sensitivity was 5/8 (62.5%).
Testing all returnees—with follow-up for disease onset and course—enabled us to evaluate the spectrum of severity for SARS-CoV-2 infections (Table). From least to most severe, 4 patients experienced asymptomatic infection, 2 mild illness, and 6 pneumonia. Prospective monitoring proved essential because of the 7 prevalent infections at entry, 5 were asymptomatic, 1 mild, and 1 pneumonia. Even with potential underascertainment of asymptomatic cases because of a lack of serologic assessment (5,6) (i.e., interval-censoring during screening tests), it is noteworthy that 4/12 persons with infections were asymptomatic. Although numbers are small, severity seemed to be age dependent (Table). No infections were detected among the 101 persons <30 years of age; of the 2 infections detected among the 136 persons 30–39 years of age, both persons were asymptomatic. Although no person in this study died, only 1 was >69 years of age. Regarding sex, excluding 1 returnee for whom sex was unknown and 14 for whom exit-screening results are pending, of the remaining 551 returnees, 9 (1.8%) of the 506 male passengers (2 asymptomatic, 2 mild, 5 pneumonia) and 3 (6.7%) of the 45 female passengers (2 asymptomatic, 1 pneumonia) were infected.
Our findings have public health implications. As recently reported (1), we found that symptom-based screening performed poorly, missing asymptomatic and presymptomatic cases. Even with universal screening, nearly half of cases were missed. Because an asymptomatic case was detected at exit screening, limiting testing of quarantined persons to those with signs/symptoms would have missed such a case; with exit-screening results pending for 14 returnees, sensitivity could be lower. The poor sensitivity of single-point testing highlights the challenges of detecting SARS-CoV-2 infections.
The potentially long incubation period of COVID-19 was consistent with that recently reported (7,8) and contributed to the large proportion of missed cases. Active daily monitoring ensured that specific illness-onset times were captured, protected from the limitations associated with patient recall of symptom onset (9). Although exposure to SARS-CoV-2 occurred at some time before quarantine (i.e., left-censored), our setting enabled us to estimate the minimum incubation period for each incident symptomatic case by taking the return date as the exposure time. Determining the specific exposure time can be difficult and is conditional according to the definition of contact. Given such qualifications, a conservative minimum incubation period of 10 days obtained prospectively in a clean quarantine setting, without recall or assumptions regarding transmission modes, is noteworthy.
Testing and follow-up of all returnees provided valuable information about the spectrum of SARS-CoV-2 infection. Most reported data have been from medically attended patients, skewed toward symptomatic patients and more severe cases, limiting our knowledge of the clinical spectrum of infection (5,10). In our setting, we could remove the influence of patients’ health-seeking behaviors and clinicians’ diagnostic practices and found that 4/12 case-patients were asymptomatic. At the same time, of the 8 case-patients who experienced symptoms, pneumonia developed in 6. Our findings were also consistent with the reported age-dependent nature of COVID-19 (2,11–13); infection and clinical attack rates were lower among younger persons. Shedding light on the severity pyramid among those infected—not only among those who sought care—provides an evidence base for risk communication, healthcare planning, and public health response. Combined with reports suggesting transmissibility of SARS-CoV-2 from asymptomatic/subclinical case-patients (1,9,14,15), our findings suggest that controlling COVID-19 through the usual tools of syndrome-based surveillance and contact tracing alone may be difficult.
When confronted with an emerging pathogen, quarantined populations can generate critical epidemiologic information. As with the First Few X study (6), our design is protected from the usual biases of passively reported surveillance data. Aggregating high-quality data from these types of investigations can build a larger severity pyramid, enabling reliable estimation of various severity measures (e.g., symptomatic proportion of infected case-patients, case severity proportion among those who are symptomatic). We recommend use of similar assessments to help elucidate the epidemiology of SARS-CoV-2 and inform public health response.
Dr. Arima is an affiliate researcher at the Infectious Disease Surveillance Center, National Institute of Infectious Diseases in Tokyo, Japan. His research interests include infectious disease epidemiology and surveillance in the context of public health practice.
Acknowledgments
We acknowledge the dedicated work of the frontline clinicians and the staff at the quarantine facilities, the workers at the local public health centers and prefectural and municipal public health institutes, and the Japan Ministry of Health, Labour and Welfare. We also thank the quarantined returnees for their patience and participation.
This study was supported in part by a grant-in aid from the Japan Agency for Medical Research and Development (grant nos. JP19fk0108104 and JP19fk0108110).
References
- Hoehl S, Rabenau H, Berger A, Kortenbusch M, Cinatl J, Bojkova D, et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382:1278–80.
- Jernigan DB; CDC COVID-19 Response Team. Update: public health response to the coronavirus disease 2019 outbreak—United States, February 24, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:216–9.
- Ng OT, Marimuthu K, Chia PY, Koh V, Chiew CJ, De Wang L, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476–8.
- Shirato K, Nao N, Katano H, Takayama I, Saito S, Kato F, et al. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis. 2020; Epub ahead of print.
- Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of COVID-19–studies needed. N Engl J Med. 2020;382:1194–6.
- World Health Organization. The First Few X (FFX) Cases and contact investigation protocol for 2019-novel coronavirus (2019-nCoV) infection [cited 2020 Mar 23].
- Morens DM, Daszak P, Taubenberger JK. Escaping Pandora’s box—another novel coronavirus. N Engl J Med. 2020;382:1293–5.
- Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020;25:25.
- Tong ZD, Tang A, Li KF, Li P, Wang HL, Yi JP, et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg Infect Dis. 2020;26:1050–2.
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020;382:692–4.
- World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) [cited 2020 Mar 23].
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;
NEJMoa2002032 ; Epub ahead of print. - Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–9.
- Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–1.
Figure
Table
Suggested citation for this article: Arima Y, Shimada T, Suzuki M, Suzuki T, KobayashiY, Tsuchihashi Y, et al. Severe acute respiratory syndrome coronavirus 2 infection among returnees to Japan from Wuhan, China, 2020. Emerg Infect Dis. 2020 Jul [date cited]. https://doi.org/10.3201/eid2607.200994
Original Publication Date: 4/10/2020





















.png)












No hay comentarios:
Publicar un comentario