A new DRUG TRIALS SNAPSHOT is now available.

TRIKAFTA is a drug for the treatment of cystic fibrosis (CF) in patients 12 years and older, who have the most common CF mutation (F508del mutation in the cystic fibrosis transmembrane conductance regulator [CFTR] gene).
CF is a rare and serious genetic disorder that results in the formation of thick mucus that builds up in the lungs and other parts of the body. This can lead to severe breathing problems.
TRIKAFTA treatment comes in co-packaged orange and blue tablets. Two orange tablets (containing a combination of three drugs elexacaftor, tezacaftor and ivacaftor) are taken by mouth in the morning. One light blue tablet (containing ivacaftor) is taken by mouth, in the evening.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: TRIKAFTA
TRIKAFTA (elexacaftor/tezacaftor/ivacaftor; ivacaftor)
(tri-KAF-tuh)
Vertex Pharmaceuticals
Approval date: October 21, 2019
(tri-KAF-tuh)
Vertex Pharmaceuticals
Approval date: October 21, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TRIKAFTA is a drug for the treatment of cystic fibrosis (CF) in patients 12 years and older, who have the most common CF mutation (F508del mutation in the cystic fibrosis transmembrane conductance regulator [CFTR] gene).
CF is a rare and serious genetic disorder that results in the formation of thick mucus that builds up in the lungs and other parts of the body. This can lead to severe breathing problems.
How is this drug used?
TRIKAFTA treatment comes in co-packaged orange and blue tablets. Two orange tablets (containing a combination of three drugs elexacaftor, tezacaftor and ivacaftor) are taken by mouth in the morning. One light blue tablet (containing ivacaftor) is taken by mouth, in the evening.
What are the benefits of this drug?
TRIKAFTA improved lung function by allowing air to move easier. In patients treated with TRIKAFTA the amount of air that can be forcibly blown out in one second [percent predicted forced expiratory volume (ppFEV1)] increased.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TRIKAFTA worked similarly in males and females.
- Race: Most of the patients were White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
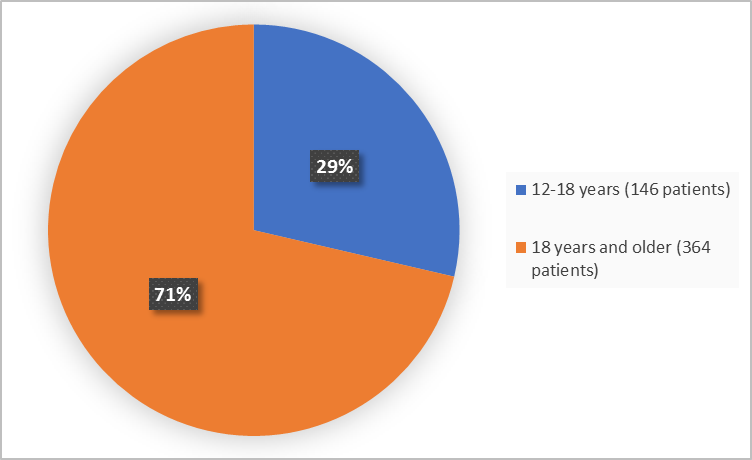
- Age: TRIKAFTA worked similarly in patients younger and older than 18 years of age.
What are the possible side effects?
TRIKAFTA may cause serious side effects including increased liver enzymes and clouding of the lens in the eye (cataracts).
The most commonly reported side effects associated with TRIKAFTA are headache, upper respiratory infections, abdominal pain, diarrhea, rash and elevated liver enzymes.
Were there any differences in side effects among sex, race and age?
- Sex: The overall occurrence of side effects was similar in males and females, with exception of headache and rash side effects, which occurred more frequently in females.
- Race: Most of the patients in the trials were White. Differences in the occurrence of side effects among races could not be determined, because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients less than and older than 18 years of age.
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved TRIKAFTA based on evidence from 2 clinical trials (Trial 1/NCT03525444 and Trial 2/NCT03525548) of 510 patients 12-64 years of age with cystic fibrosis and at least one F508del mutation.
Trials were conducted at 154 sites in USA, Canada, Austria, Belgium, Czech Republic, France, Germany, Greece, Italy, Netherlands, Sweden, UK and Australia.
Figures 1 – 3 below summarize by sex, race and age how many patients participated in the combined clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Efficacy Trials by Race
Race | Number of Patients | Percentage of Patients |
White | 473 | 93 |
Black or African American | 4 | 1 |
All Other | 7 | 1 |
Not Collected* | 26 | 5 |
*Data not collected due to local regulations
FDA Review
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
The benefit and side effects of TRIKAFTA were evaluated in two trials.
Trial 1 was conducted in CF patients, who have one copy of the F508del mutation. Patients were randomly assigned to receive TRIKAFTA, or placebo twice daily for 24 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
Trial 2 was conducted in CF patients who have 2 copies of F508del mutation. Patients were randomly assigned to receive TRIKAFTA, or an active control (an approved CF drug tezacaftor/ivacaftor) twice daily for 4 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
In both trials, the benefit of TRIKAFTA was assessed by change in ppFEV1, the amount of air that can be forcibly blown out in one second. In Trial 1 TRIKAFTA was compared to placebo after 4 weeks of treatment and in Trial 2 to active comparator after 4 weeks of treatment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.




































No hay comentarios:
Publicar un comentario