
Bone Marrow Transplant Drug May Improve Immunotherapy for Metastatic Breast Cancer
March 11, 2019, by NCI Staff
Some types of cancer cells are surrounded by a dense layer of connective tissue that acts as a barrier to cancer-killing immune cells. That poses a problem for immunotherapies, treatments that depend on interactions between immune cells and cancer cells.
But a new NCI-funded study in mouse models of metastatic breast cancer suggests that plerixafor (Mozobil)—a drug already used for bone marrow transplants—can thin this protective tissue layer and allow more immune cells to reach the tumors.
The results, published January 30 in the Proceedings of the National Academy of Sciences, also show that treating mice with plerixafor improved how well immune checkpoint inhibitors, a common type of immunotherapy, worked to decrease metastasis and prolong survival.
Given that immune checkpoint inhibitors don’t work well for most women with metastatic breast cancer, these findings suggest that adding plerixafor might be a way to boost the efficacy of these treatments, wrote the lead investigator, Rakesh Jain, Ph.D., of Harvard Medical School and Massachusetts General Hospital, and his colleagues.
There are multiple ongoing clinical trials of plerixafor for people with different types of cancer, and a trial for people with breast cancer is being explored, Dr. Jain noted. In several of these studies, the safety of the treatment, as well as how it affects immune cells within the tumor microenvironment, will be assessed.
But dense connective tissue may not be the only reason why checkpoint inhibitors are ineffective for some women with breast cancer, noted George Sledge, M.D., of Stanford University, in an accompanying commentary. Nevertheless, he wrote, “new approaches are still needed if we are to optimize checkpoint inhibitor therapy,” and the results of this study “offer a clinically testable hypothesis.”
The study “touches on many different layers of complexity in cancer development and progression and how to think about targeting tumors in a more effective manner,” said Jeffrey Hildesheim, Ph.D., chief of the Tumor Biology and Microenvironment Branch of NCI’s Division of Cancer Biology, who wasn’t involved in the study.
Fibroblasts Keep Immune Cells Out
There are many factors that can affect how the immune system responds to cancer. One key player is a kind of cell known as a fibroblast.
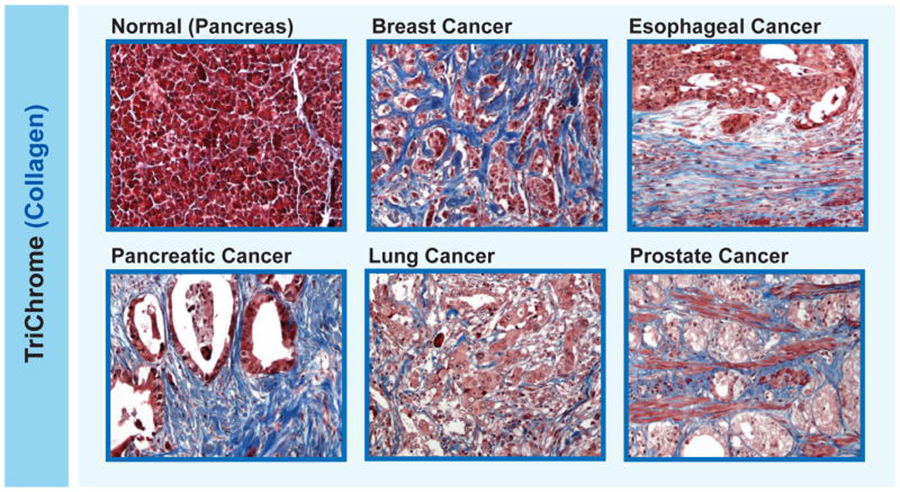
In the tumor microenvironment, fibroblasts create a meshwork of connective fibers (such as collagen) that helps support the tumor. But when cancer-associated fibroblasts overproduce this connective tissue, it can physically prevent immune cells from reaching the tumor.
The overgrowth of connective tissue around cancer cells (what’s called desmoplasia) can compress blood vessels and limit how much oxygen gets to the tumor. Low oxygen, in turn, dampens the activity of cancer-killing immune cells.
On top of that, fibroblasts produce proteins called cytokines that mediate the recruitment and activation of different kinds of immune cells. These cytokines “may be attracting the wrong crowd to the tumor,” Dr. Hildesheim explained. Meaning, they may recruit certain immune cells that help the tumor grow and spread while blocking immune cells that may destroy it.
Desmoplasia is a common feature of certain types of cancer, including pancreatic, esophageal, prostate, and some types of breast cancer. Some researchers believe that desmoplasia is partially to blame for the limited success of immunotherapies in people with these kinds of cancer.
Do Metastatic Breast Tumors Have Desmoplasia?
Because primary tumors in the breast can be desmoplastic, Dr. Jain and his colleagues investigated whether that was also the case for breast tumors that had spread to other organs.
“Primary and metastatic cancer are not one in the same and they need to be studied more carefully as their own entities,” Dr. Hildesheim said. That’s an important point of this study, he added.
First, the researchers examined paired samples of primary and metastatic tumors from 17 women with breast cancer that had spread to their liver or lungs. Both primary and metastatic tumors were desmoplastic, and the metastatic tumors had nearly no cancer-killing immune cells, they found.
The scientists then sifted through data from The Cancer Genome Atlas, searching for genes that were present at high levels in breast tumors and correlated with the exclusion of immune cells.
Among the genes in that category was CXCL12, which makes a protein that influences the tumor microenvironment in different ways. For example, when the CXCL12 protein binds to fibroblasts, the cells migrate toward the source of CXCL12 and produce more collagen. Different kinds of cells produce CXCL12, including cancer cells and fibroblasts.
The researchers discovered that the paired primary and metastatic breast tumors had higher levels of the CXCL12 receptor, CXCR4, than nearby healthy tissues.
Targeting CXCR4 with Plerixafor
Next, the investigators determined whether inhibiting CXCR4 could reverse desmoplasia and the exclusion of cancer-killing immune cells.
In a mouse model of metastatic breast cancer that mimicked the desmoplasia observed in human tumors, they found that cancer-associated fibroblasts from primary and metastatic tumors had high levels of CXCR4.
When the team treated the mice with plerixafor—which inhibits CXCR4—it reduced desmoplasia and blood vessel compression, leading to more oxygen in the tumors, compared with a control treatment.
Plerixafor treatment also led to an increase in levels of cytokines that activate the immune system and a decrease in levels of proteins that aid breast cancer metastasis.
In addition, there were fewer metastatic tumors in the lungs of mice treated with plerixafor and these mice lived longer than mice treated with a placebo.
Enhancing the Effects of Immunotherapy
The research team then checked whether plerixafor could improve the response to immune checkpoint inhibitors in three mouse models of metastatic breast cancer.
Earlier studies from Dr. Jain’s team and others have shown that plerixafor enhances responses to immunotherapy in mouse models of pancreatic cancer and liver cancer.
After the scientists surgically removed the primary tumors and the mice developed metastases, they were given one of four treatments: a control, plerixafor, a combination of immune checkpoint inhibitors, or plerixafor plus the immune checkpoint inhibitors.
Compared with the other treatments, plerixafor plus the checkpoint inhibitors led to fewer cancer-associated fibroblasts, less desmoplasia, and more cancer-killing immune cells in the metastatic tumors. The combination of plerixafor and checkpoint inhibitors also decreased lung metastases and extended how long the mice lived.
In all three models, the researchers noted, several mice treated with plerixafor and checkpoint inhibitors had long-term remissions, doubling the rate of long-term remissions observed for immune checkpoint inhibitors alone.
Several different CXCR4 inhibitors are currently available and they are “relatively nontoxic,” Dr. Sledge noted.
Down the road, the basic biology findings from this study may help guide clinical studies of CXCR4 inhibitors in combination with other therapies, said Dr. Hildesheim.





















.png)












No hay comentarios:
Publicar un comentario