Childhood Cancers Research
Why Research Is Critical to Progress against Childhood Cancer
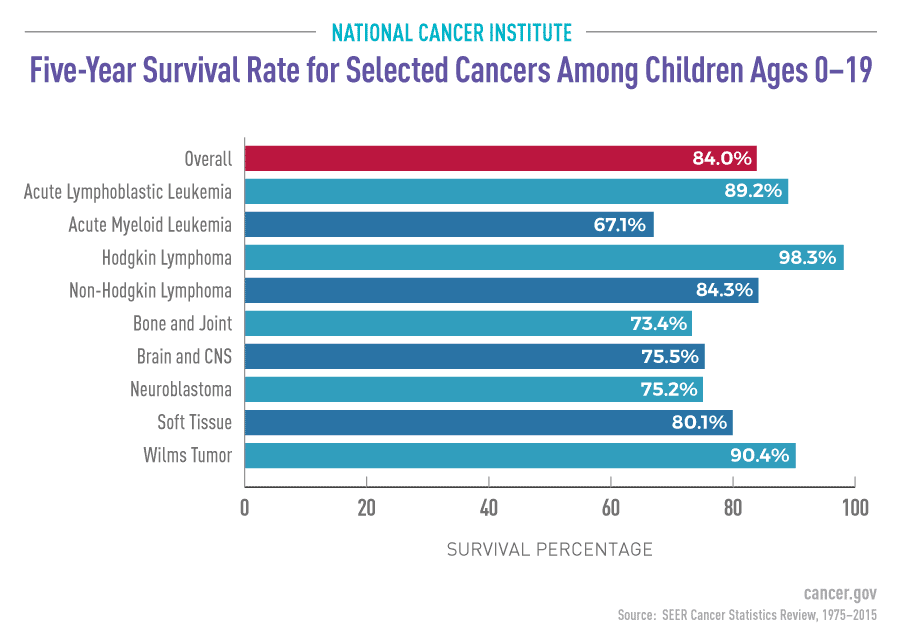
Cancer is the leading cause of death from disease among children and adolescents in the United States. Although substantial progress has been made in the treatment of several types of childhood cancer over the past 5 decades, progress against other types has been limited. Even when long-term survival is achieved, many survivors of childhood cancer may experience long-term adverse effects from the disease or its treatment. Clearly, more research is needed to develop new, more-effective, and safer treatments for childhood cancer. Learn more about research directions for childhood cancer.
NCI has a number of programs that address childhood cancers specifically, and many of the institute’s other research programs are applicable to children with cancer even if they aren’t focused specifically on pediatric cancers. The institute supports a broad range of biomedical research that is relevant to this population, including:
- Basic research to enhance our understanding of the fundamental mechanisms of cancer
- Clinical research to test new treatments for safety and effectiveness
- Survivorship research to reduce the long-term adverse effects of cancer and its treatment
Challenges in Childhood Cancer Research
One challenge in conducting research on childhood cancer is that cancers in children and adolescents are relatively uncommon. Childhood cancers represent less than 1% of all new cases of cancer diagnosed in the United States each year. As clinical trials are increasingly restricted to smaller numbers of patients who are defined by the molecular characteristics of their tumors, rather than where the tumors originated in the body, collaboration among children’s cancer centers and a strong national clinical research program will continue to be essential to ensure that trials enroll sufficient numbers of participants to produce meaningful results.
Another challenge is that very little is known about the causes of childhood cancers. A small percentage of cancers in children and adolescents can be linked to inherited genetic abnormalities or exposures to diagnostic or therapeutic radiation, but the role of environmental exposures, including infectious agents and toxic chemicals, is unclear. As a result, identifying opportunities to prevent childhood cancer may be difficult.
In addition, the types of cancers children develop, and the biology of those cancers, generally differ from those of cancers diagnosed in adults. For example, tumors of developing organs and tissues (such as retinoblastomas in the eye and osteosarcomas in bone) are more common in children.
Moreover, most childhood cancers have relatively few genetic alterations, and they often lack the genetic targets for treatments that have been developed and approved for cancers occurring in adults. And drugs that target signaling pathways that are active in some adult cancers might be difficult to use in children, given that many of these signaling pathways are essential for normal development.
In fact, childhood cancers are often driven by genetic alterations that are distinct from those that occur in adult cancers. As an example, some childhood cancers are initiated by fusion genes that result from chromosomal translocations that produce "fusion oncoproteins." Few treatments have been developed to date that target these types of cancer-causing genetic alterations. Another contributing factor to the small number of targeted therapies for childhood cancers is that the rarity of these diseases has been an impediment to commercial drug development.
Additional challenges in childhood cancer research are developing new treatments that are less toxic and cause fewer adverse effects (both acute and late) than current treatments and developing interventions to mitigate the adverse effects of both current and future treatments. The late effects of childhood cancer therapy can have profound physical, emotional, and other consequences for survivors, including a shortened life expectancy. How to minimize and address these late effects to improve both the quality and the length of life of survivors is a research priority.
More information about drug metabolism in children, which varies with developmental age, is also needed, as are better laboratory and animal models for screening and testing drugs for potential use in children and adolescents. The optimal use of radiation therapy in treating childhood cancers also needs to be defined so that efficacy is maintained or increased while long-term side effects are reduced.
Basic Research Drives Progress against Childhood Cancer
Virtually all progress against cancer in both children and adults has its origins in basic research, often in areas that are not directly related to the disease.
As an example, the discovery of the CRISPR/Cas system for gene editing has revolutionized the study of genes that control cancer cell growth and survival in both childhood and adult cancers. This discovery came from basic research in microbiology on the mechanisms by which bacteria resist infections by viruses.
Another example had its origins in basic research on proteins called histones, which are DNA-binding proteins that provide structural support for chromosomes and help control the activity of genes. Scientists spent years investigating how these proteins are modified in the cell nucleus and the role of histone modifications in controlling when and to what extent genes are expressed.
The findings of this research became immediately relevant to a type of pediatric brain tumor called diffuse intrinsic pontine glioma (DIPG) when it was discovered that most DIPG tumors have a mutation in the gene for the histone protein H3.3 that prevents a specific modification of the protein. This mutation in H3.3 is thought to be a driver mutation for DIPG and is associated with aggressive disease and shorter survival.
How NCI Programs Are Making a Difference in Childhood Cancer
NCI recognizes that children and adolescents are not just small adults and that specialized treatments tailored to childhood cancers are needed. Therefore, NCI supports an array of programs specifically to advance childhood cancer care, and has renewed these initiatives and programs over numerous funding periods. Some of these programs include:
- The Pediatric Oncology Branch (POB) in NCI’s Center for Cancer Research conducts high-risk, high-impact basic, translational, and clinical research on childhood cancers. For example, POB Chief Brigitte Widemann, M.D., recently led the only treatment trial in the nation for children with tumors caused by neurofibromatosis type 1 (NF1), a genetic disorder in which painful and often disfiguring tumors of the nerves can grow on or under the skin. The phase I trial showed that children tolerated the drug selumetinib, and nearly all experienced tumor shrinkage.
- The Children's Oncology Group (COG), which is part of NCI's National Clinical Trials Network (NCTN), develops and coordinates pediatric cancer clinical trials that are available at more than 200 member institutions, including cancer centers throughout the United States and Canada. In addition to conducting traditional late-phase clinical trials, COG has established a Phase 1 and Pilot Consortium to conduct early-phase trials and pilot studies so new anticancer agents can be rapidly and efficiently introduced into pediatric cancer care.
- The NCI–COG Molecular Analysis for Therapy Choice (Pediatric MATCH) precision medicine trial is a nationwide trial to explore whether targeted therapies can be effective for children and adolescents with solid tumors that harbor specific genetic mutations and have progressed during or after standard therapy. The trial which is funded by NCI and conducted by COG opened to patient enrollment on July 24, 2017. Germline testing is performed on all patients enrolled to assess whether the genetic aberrations identified in their tumors are inherited. The genomic data captured in the trial will serve as an invaluable resource for researchers seeking to understand the genetic basis of pediatric cancer.
- The Pediatric Brain Tumor Consortium (PBTC) is a multidisciplinary cooperative research organization devoted to identifying better treatment strategies for children with primary brain tumors.
- The Childhood Cancer Survivor Study (CCSS) is examining the long-term adverse effects of cancer and cancer therapy on approximately 35,000 survivors of childhood cancer who were diagnosed between 1970 and 1999. The study was created to gain new knowledge about the long-term effects of cancer and its treatment, and to educate survivors and the medical community about the potential impacts of a cancer diagnosis and treatment. The results obtained from CCSS are used to help design treatment protocols and interventions that will result in an increase in survival, while minimizing the harmful late effects. This research is also used to develop and expand programs for early detection and prevention of late effects in children and adolescent cancer survivors.
- The New Approaches to Neuroblastoma Therapy (NANT) Consortium consists of a multidisciplinary team of laboratory and clinical scientists focused on improving outcomes for patients with high-risk neuroblastoma by discovering mechanisms of resistance to therapies, discovering targetable vulnerabilities driving resistance, and translating these insights into clinical trials. NANT works closely with COG to translate their experimental therapy findings into COG phase III clinical trials. Their findings regarding the tumor microenvironment, tumor response to therapy, and the application of cellular therapies to solid tumors have implications beyond neuroblastoma.
- The Pediatric Preclinical Testing Consortium (PPTC) systematically evaluates new agents in genomically characterized models of childhood cancer. The primary goal of the PPTC is to develop high-quality preclinical data to help pediatric oncology researchers identify agents that are most likely to show significant anticancer activity when tested in the clinic against selected childhood cancers.
- The Therapeutically Applicable Research to Generate Effective Treatments (TARGET)program uses genomic approaches to catalog the molecular changes in several types of childhood cancer to increase our understanding of their pathogenesis, improve their diagnosis and classification, and identify new candidate molecular targets for better treatments. For example, TARGET researchers performed a pan-cancer study of somatic alterations in nearly 1,700 pediatric leukemias and solid tumors and found major genomic differences when compared with adult cancers. The related Cancer Genome Characterization Initiative includes genomic studies of various pediatric cancers that often do not respond well to treatment.
- The Hyperactive RAS Specialized Programs of Research Excellence (SPOREs) focus on developing better treatments for neurofibromatosis type 1 and related cancers in children, adolescents, and young adults.
- NCI held two workshops in 2015, as part of the NCI Provocative Questions Initiative, to identify questions that address gaps in the pediatric oncology research field. NCI will release program announcements and funding opportunities for these pediatric oncology provocative questions, which are intended to generate innovative approaches to childhood cancer research challenges.
- As part of the Cancer Moonshot, NCI is planning to establish the Fusion Oncoproteins in Childhood Cancers (FusOnC2) Consortium in 2018. This multidisciplinary, collaborative network of investigators will focus their research on select fusion oncoproteins implicated in childhood cancers that have a high risk of treatment failure and for which there has been little progress in identifying targeted agents.
- Also as part of the Cancer Moonshot, NCI is planning to establish a Pediatric Immunotherapy Discovery and Development Network (PI-DDN) in 2018. This collaborative research network will work to identify and advance research opportunities for translating immunotherapy concepts for children and adolescents with cancer toward clinical applications. Primary goals of the PI-DDN will include the discovery and characterization of immunotherapy targets for childhood and adolescent cancers, the development of new immunotherapy treatment approaches, and an improved understanding of the immunosuppressive tumor microenvironment in order to advance new, more effective immune-based treatment regimens for high-risk pediatric cancers.
- The Pediatric Cancer Immunotherapy Trials Network (CITN) is utilizing the clinical trials infrastructure of the CITN so that it can conduct clinical trials of immunotherapy agents of specific relevance to children and adolescents with cancer. Examples of the types of novel treatments to be investigated by the Pediatric CITN include cellular therapies (e.g., CAR T cells targeting pediatric cancer antigens) and antibody-based therapies, including antibody-drug conjugates, that target surface antigens preferentially expressed on childhood cancers.




































No hay comentarios:
Publicar un comentario