| |||||||||||
Volume 24, Number 6—June 2018
Research
Genomic Epidemiology of Global Carbapenemase-Producing Enterobacter spp., 2008–2014
On This Page
Gisele Peirano1, Yasufumi Matsumura1, Mark D. Adams2, Patricia Bradford, Mary Motyl, Liang Chen, Barry N. Kreiswirth, and Johann D.D. Pitout
Abstract
We performed whole-genome sequencing on 170 clinical carbapenemase-producing Enterobacter spp. isolates collected globally during 2008–2014. The most common carbapenemase was VIM, followed by New Delhi metallo-β-lactamase (NDM), Klebsiella pneumoniae carbapenemase, oxacillin 48, and IMP. The isolates were of predominantly 2 species (E. xiangfangensis and E. hormaechei subsp. steigerwaltii) and 4 global clones (sequence type [ST] 114, ST93, ST90, and ST78) with different clades within ST114 and ST90. Particular genetic structures surrounding carbapenemase genes were circulating locally in various institutions within the same or between different STs in Greece, Guatemala, Italy, Spain, Serbia, and Vietnam. We found a common NDM genetic structure (NDM-GE-U.S.), previously described on pNDM-U.S. from Klebsiella pneumoniae ATCC BAA-214, in 14 different clones obtained from 6 countries spanning 4 continents. Our study highlights the importance of surveillance programs using whole-genome sequencing in providing insight into the molecular epidemiology of carbapenemase-producing Enterobacter spp.
The emergence of carbapenem resistance is a major public health concern because these agents are regarded as one of the last effective therapies available for treating serious infections caused by Enterobacteriaceae (1). Carbapenemases are important causes of carbapenem resistance because they can be transferred between members of the Enterobacteriaceae. The most common carbapenemases among clinical Enterobacteriaceae are the Klebsiella pneumoniae carbapenemases (KPCs) (Amber class A), IMPs, VIMs, New Delhi metallo-β-lactamase (NDMs) (class B or metallo-β-lactamases), and oxacillin (OXA) 48–like (class D) enzymes (2).
Recent surveillance studies have shown that Enterobacter spp. are often the second or third most common Enterobacteriaceae species associated with carbapenemases (3,4). Typically, KPCs are common among Enterobacter spp. from the United States and South America (5). VIMs are often limited to Europe, NDMs to the Indian subcontinent, and OXA-48 to North Africa and the Middle East (5).
Comprehensive global data regarding the different Enterobacter species and molecular epidemiology are currently limited. We designed a study that used short-read whole-genome sequencing to describe the molecular characteristics and international distribution of Enterobacter spp. with different carbapenemases (n = 170) obtained from 2 global surveillance systems during 2008–2014.
Bacterial Isolates
We included 170 clinical, nonrepeat Enterobacter spp. collected from 2 global surveillance programs, namely the Merck Study for Monitoring Antimicrobial Resistance Trends (SMART) (2008–2014) and the AstraZeneca global surveillance program (2012–2014), presently known as the INFORM Global Surveillance Study of Antimicrobial Resistance (Technical Appendix 1 Table 1; Technical Appendix 2). The isolates initially underwent phenotypic identification and microdilution panel susceptibility testing, and all carbapenem-nonsusceptible isolates underwent molecular screening for blaKPC, blaVIM, blaNDM, blaOXA-48–like, blaIMP, and blaGES as described previously (6). We obtained a total of 142,226 Enterobacteriaceae from the period 2008–2014, and 6,457 (4.5%) were identified as Enterobacter spp.; 682 were nonsusceptible to 1 of the carbapenems, and 170/6,457 (2.6%) were positive for blaKPC, blaOXA-48–like, blaNDM, blaVIM, and blaIMP and thus included in our study.
Whole-Genome Sequencing
We used the Nextera XT DNA sample preparation kit (Illumina, San Diego, CA, USA) to prepare libraries for sequencing. We multiplexed and sequenced samples on an Illumina NextSeq500 for 300 cycles (151 bp paired-end).
Genomic Analysis
We obtained draft genomes by using SPAdes version 3.10.1 (7). We identified species based on the hsp60 gene sequences (8). We created whole-genome phylogenetic trees, including reference strains for identification of E. cloacae complex (9; Technical Appendix 1 Table 2).
To define the presence of genes and their alleles, we accessed BLAST in combination with the following databases or typing schemes: BLAST (http://blast.ncbi.nlm.nih.gov/Blast), National Center for Biotechnology Information (NCBI) Beta-Lactamase Data Resources (http://www.ncbi.nlm.nih.gov/pathogens/beta-lactamase-data-resources), ResFinder (10), PlasmidFinder (11), and Enterobacter cloacae Multilocus Sequence Typing (MLST) Databases (http://pubmlst.org/ecloacae). We classified integrons according to INTEGRALL (http://integrall.bio.ua.pt).
Phylogenetic Analysis
We created a recombination-free, core single-nucleotide polymorphism (SNP)–based phylogenetic tree and identified SNPs by mapping the reads or aligning the genomes against E. xiangfangensis type strain LMG27195 (9) using the RedDog pipeline (https://github.com/katholt/RedDog). We removed recombination sites according to Gubbins (12) and removed prophages identified by PHAST (13). We included core SNPs and sites that were present in all genomes to create a maximum-likelihood tree using RAxML with the general time-reversible plus gamma substitution model (14). We visualized the tree by using iTOL version 3 (15).
To identify clades within certain sequence types (STs), we used a phylogeny-free population genetics approach of core SNPs, conducting hierarchal clustering analysis with the Bayesian Analysis of Population Structure program (16). We included all 1,048 available Enterobacter spp. genomes in the NCBI Reference Sequence Database (http://www.ncbi.nlm.nih.gov/refseq) as of June 20, 2017. An in silico MLST analysis identified 282 STs from 950 typeable genomes. We included a total of 201 genomes of STs 78, 90, 93, 105, 108, 114, and 171 for the clustering analysis (Technical Appendix 1Table 3). For each E. hormaechei subspecies or E. xiangfangensis, the hierarchal Bayesian Analysis of Population Structure clustering analysis (16) was conducted with 3 nested levels with a priori upper bound of the number of clusters between one fourth to one half of the total number of isolates. We defined clades by using the second level of clustering.
Sequence Data Accession Numbers
We deposited the sequencing data in the DNA Data Bank of Japan and NCBI (NCBI BioProjects PRJNA259658 and PRJNA398291) databases (accession nos. DRA004879, SRP046977, and SRR2960053–SRR2960159 [SMART isolates] and SRR5939895–SRR5939952 [AstraZeneca isolates]). The sequences of new integrons or genetic environments described in this study were GenBank accession nos. LC224310–2, MF288916–351991, and MF327263–71.
Global Distribution of Carbapenemases among Enterobacter spp.
We included a total of 170 carbapenemase-producing Enterobacter strains in the study. The VIMs (VIM-1, 4, 5, 23, and 31; n = 51 [46 were only positive for VIM, and 5 co-produced OXA-48]) were the most common carbapenemase among this collection, followed by NDMs (NDM-1, 6, and 7; n = 43 [41 were positive only for NDM, 1 also co-produced OXA-48. and 1 co-produced KPC-2]); KPCs (KPC-2, 3, 4, and 5; n = 38 [37 were only positive for KPC, and 1 co-produced NDM]); OXA-48 (n = 31 [25 were only positive for OXA-48, 5 co-produced VIM, and 1 co-produced NDM]); and IMPs (IMP-1, 4, 8, 13, and 14; n = 14). Enterobacter spp. with blaVIM were mostly limited to Europe; isolates with blaNDM were present predominantly in the Balkans, India, and Vietnam; isolates with blaKPC were mainly found in the United States and South America; isolates with blaOXA-48 were largely present in North Africa and the Middle East; and isolates with blaIMP occurred mostly in the Philippines, Taiwan, and Australia. The global distribution of isolates from this study was similar to what had previously been reported for other members of Enterobacteriaceae, especially Klebsiella spp. with carbapenemases (5,17).
E. aerogenes Distant from E. cloacae Complex
We identified 10 isolates as E. aerogenes. These results are described in Technical Appendix 2.
E. xiangfangensis Identified as the Most Common Species
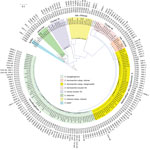
Figure 1. Phylogenetic tree of the different species and sequence types among 160 Enterobacter cloacaecomplex isolates identified from Enterobacter spp. isolates collected in the Merck Study for Monitoring Antimicrobial Resistance Trends, 2008–2014,...
The E. cloacae complex (n = 160) from our study was obtained from intraabdominal (n = 69), urine (n = 56), skin and soft tissue (n = 19), blood (n = 2), and respiratory specimens (n = 14). We identified 8 species among E. cloacae complex (E. xiangfangensis [n = 65], E. hormaechei subsp. steigerwaltii [n = 47], E. cloacae cluster III [n = 14], E. cloacae subsp. cloacae [n = 13], E. cloacae cluster IV [n = 9], E. hormaechei subsp. oharae [n = 6], Enterobacter asburiae [n = 5], and Enterobacter kobei [n = 1]). These species were associated with different types of carbapenemases and showed global distribution (Figure 1; Technical Appendix 2 Table 1). E. xiangfangensis was frequent in the Balkans (e.g., Croatia, Romania, and Serbia), whereas E. hormaechei subsp. steigerwaltii was mostly prevalent in Greece and Vietnam (Technical Appendix 2 Table 2 Table 1). This overrepresentation was attributable to the presence of particular STs among these species (Technical Appendix 2 Table 2).
Dominant Sequence Types Identified among 4 Species in E. cloacae Complex
E. xiangfangensis from our study comprised 18 different STs, including 1 dominant ST, ST114 (19/65; 29%). E. hormaechei subsp. steigerwaltii comprised 15 different STs, including 2 dominant STs, ST90 (10/47; 21%) and ST93 (14/47; 30%). E. cloacae cluster III comprised 4 different STs, including 1 dominant ST, ST78 (10/14 [71%). All 6 of the E. hormaechei subsp. oharae isolates belonged to ST108 (Figure 1). The remaining species did not contain a dominant ST, and we found new STs among E. cloacae cluster IV (ST832 and ST834) and E. cloacae subsp. cloacae (ST835, ST836, and ST837).
Major and Minor Sequence Types among Enterobacter cloacae Complex
Among the E. cloacae complex, we identified 4 major STs (>10 isolates/ST), ST114, ST93, ST90, and ST78. We also identified 2 minor STs (5–9 isolates/ST), ST105 and ST108.

Figure 2. Phylogenetic tree of the different clades among 40 Enterobacter xiangfangensis ST14 isolates identified from Enterobacter spp. isolates collected in the Merck Study for Monitoring Antimicrobial Resistance Trends, 2008–2014, and the AstraZeneca...
ST114 (n = 19) from E. xiangfangensis was the most common ST and divided into 4 clades. Isolates representing 3 of the clades (ST114A, ST114B, and ST114C) were from this study, and isolates representing clade ST114D were from a different study (9; Figure 2; Technical Appendix 2 Table 2). ST114 had a global distribution (Greece, Italy, Kuwait, Morocco, Romania, Serbia, Tunisia, and the United States) and was associated with different carbapenemases (VIM-1, VIM-4+OXA-48, NDM-1, KPC-2, and OXA-48) (Technical Appendix 2 Table 2). The largest clade (ST114A [n = 13]) was present in Serbia, Romania (with blaNDM-1), Tunisia, Morocco, and Kuwait (with blaOXA-48) (Technical Appendix 2 Table 2). Clade ST114B (n = 4) with blaVIM-1 was obtained from Greece and Italy, and clade ST114C (n = 2; 1 with blaVIM-1 and 1 with blaKPC-2) was found in the United States. ST114 is a common global human Enterobacter clone (18) and is also present in companion animals (19). This international clone is associated with various antimicrobial resistance determinants (20) and was responsible for a prolonged nosocomial outbreak involving KPC-3 in the United States (21).

Figure 3. Phylogenetic tree of the different clades among 51 Enterobacter hormaechei subsp. steigerwaltii ST90 and ST93 isolates identified from Enterobacter spp. isolates collected in the Merck Study for Monitoring Antimicrobial Resistance Trends,...
ST93 (n = 14), from E. hormaechei subsp. steigerwaltii, was the second most common ST in this collection and consisted of 1 clade (Figure 3; Technical Appendix 2 Table 2). ST93 had a global distribution (Australia, Belgium, China, Romania, Spain, Thailand, United States, and Vietnam) and was associated with different carbapenemases (IMP-8, IMP-14, VIM-1, NDM-1, KPC-2, and OXA-48). ST93 was mostly present in Vietnam (n = 7), where it contained blaNDM-1 and blaOXA-48 (Technical Appendix 2 Table 2).
ST90 (n = 10), from E. hormaechei subsp. steigerwaltii, and ST78 (n = 10), from E. cloacae cluster III, were the next most common STs in our collection. ST90 was divided into 3 clades; isolates from 2 of the clades (ST90B and ST90C) were from this collection, whereas isolates representing clade ST90A were from a different study (22; Figure 3; Technical Appendix 2 Table 2). ST90C with blaVIM-1 (n = 7) was from Greece, whereas clade ST90B showed an international distribution (ST90C with IMP-4 from Australia, KPC-2 from Canada, and NDM-1 from Romania).
ST78 from E. cloacae cluster III consisted of 1 clade. This ST was associated with VIM-1 (Greece, Italy, and Spain), IMP-4 (Philippines), IMP-8 (Taiwan), and OXA-48 (Turkey) (Technical Appendix 2 Table 2).

Figure 4. Phylogenetic tree of the different clades among 39 Enterobacter hormaechei subsp. oharae ST108 isolates identified from Enterobacterspp. isolates collected in the Merck Study for Monitoring Antimicrobial Resistance Trends, 2008–2014, and...
The minor STs, including ST105 and ST108 (both with 6 isolates), were distinguished on the basis of their molecular epidemiology. ST105 from E. xiangfangensis belonged to a single clade and was only present in Croatia, where it contained blaVIM-1. All the E. hormaechei subsp. oharae isolates belonged to ST108, which was divided into 5 clades; isolates from 2 of the clades (ST108C and ST108D) were from this collection, whereas isolates representing the other clades were from different studies (23; Figure 4). Clade 108C (n = 4) was present in Spain with blaVIM-1 (n = 2) and China with blaIMP-1 (n = 2), and ST108D (n = 2) was found in Australia (with blaIMP-4) and Israel (with blaOXA-48).
β-lactamases, Antimicrobial Resistance Determinants, and Plasmid Analysis
For each of the 170 isolates, we tabulated the study number, GenBank accession number, species, date, country of isolation, ST, and clade. The β-lactamases, antimicrobial resistance determinants, plasmid replicon types, and plasmid STs are shown in Technical Appendix 1 Table 1 and Technical Appendix 2.
Genetic Environments Surrounding the Carbapenemase Genes
We were able to successfully characterize the immediate genetic environments surrounding the carbapenemase genes in 8/14 E. cloacae complex with IMP, 28/33 with KPC (including 4 novel structures named KPC-GE01, KPC-GE02, KPC-GE03, and KPC-GE04), 42/42 with NDM (including 4 novel structures named NDM-GE01, NDM-GE02, NDM-GE03, and NDM-GE04), 17/27 with OXA-48 (including 4 novel structures named OXA-GE01, OXA-GE02, OXA-GE03, and OXA-GE04), and 46/51 with VIM (including the novel integrons In1372, In1373, and In1374) (Technical Appendix 2 Table 3). We have also described the novel structures found in E. aerogenes (Technical Appendix 2).
The blaKPC were mainly associated with the Tn4401b isoform (including the 4 novel structures), whereas blaOXA-48 was always associated with Tn1999(including the 4 novel structures). Isolates with NDM contained ISAba125 upstream and bleMBL downstream of the blaNDM, and the blaVIM and blaIMPwere situated within diverse class I integrons from various countries (Technical Appendix 2 Table 2).
Integrons Harboring blaVIM-1 Circulating Locally within the Same or between Different STs in Spain, Greece, and Italy
In237 was present in ST78 (obtained in 2013) and ST90C (obtained in 2014) from the same institution in Greece. In916 was identified in ST78 (obtained in 2010) and ST114B (obtained in 2014) from the same institution in Italy. In624 was harbored in ST78, ST96, and ST108 from the same institution in Spain (all obtained in 2010). In87 was detected in ST98, ST110, and ST141 from 2 different institutions in Greece (obtained in 2010 and 2014). In4873 was identified in ST114B from 2 different institutions in Greece (obtained in 2013) (Technical Appendix 2 Tables 1, 2). In110 with blaVIM-1 was present in ST105 from Croatia (obtained in 2013) and ST520 from Spain (obtained in 2012).
Global Distribution of a Common NDM-1 Genetic Structure
The most common genetic structure immediately surrounding the blaNDMs (named NDM-GE-U.S.) in our collection was identical to that previously described on a 140.8 kb IncA/C plasmid (pNDM-U.S.; GenBank accession no. CP006661.1) found in K. pneumoniae ATCC BAA-2146 with blaNDM-1 (24). This bacterium was isolated in 2010 from the urine of a US hospital patient who had previously received medical care in India (25). NDM-GE-U.S., a 3,063-bp fragment consisting of ΔISAba125-blaNDM-1-bleMBL-trpF-dsbC, was present in 16/42 of NDM E. cloacae complex isolates among 14 different STs (88, 90B, 93, 114A, 279, 136, 182, 270, 435, 513, 524, 525, 609, and 832) obtained from Colombia, Romania, Philippines, Vietnam, South Africa, and Kenya (Technical Appendix 2 Table 3).
We determined the sequence similarity of the isolates with NDM-GE-U.S. to previously sequenced plasmids in the GenBank database. The similarity to pNDM-U.S. ranged from 7% to 81%, suggesting that different plasmids contained NDM-GE-U.S. Twelve of the isolates showed high similarity (range 93%–100%) to pK518_NDM1, a 106.8-kb IncFII plasmid with blaNDM-1 from China (GenBank accession no. CP023187). The remaining 4 isolates showed high similarity (range 98%–100%) with the 54-kb IncX3 plasmid pNDM-HN380 (n = 3) from China (26) and the 178.2-kb IncA/C plasmid p6234–178.193kb (n = 1) from the United States (GenBank accession no. CP010391).
The most common carbapenemase among Enterobacter spp. from our study was VIM, followed by NDM, KPC, OXA-48, and IMP. Carbapenemase-producing Enterobacter spp. was dominated by 2 global species, namely E. xiangfangensis with 1 major clone (ST114) and E. hormaechei subsp. steigerwaltii with 2 major clones (ST90 and ST93). ST114 and ST90 were divided into different clades; some of the clades (e.g., 90C and 114B) were located in certain geographic regions affiliated with specific carbapenemases, whereas other clades (114A and 90B) were distributed globally in association with different types of carbapenemases.
The taxonomy of E. cloacae complex is confusing, and uncertainty still remains about what species make up this complex. In the early 2000s, Hoffmann and Roggenkamp (8) sequenced hsp60 and established 12 genetic clusters (I to XII) in E. cloacae complex. In 2005, the same authors further defined the taxonomy of E. cloacae complex and named cluster VII as E. hormaechei subsp. hormaechei, cluster VI as E. hormaechei subsp. oharae, and cluster VIII as E. hormaechei subsp. steigerwaltii (27). In 2014, Gu et al. (28) described a novel Enterobacter species obtained from sourdough in China named E. xiangfangensis, which clustered closest to E. hormaechei.
The first study that described the global distribution of E. cloacae clones was undertaken by Izedebski et al (18), who performed MLST on 173 cephalosporin-resistant E. cloacae isolates obtained from Israel and several countries in Europe. MLST identified 88 STs among this collection, with ST78, ST114, ST108, and ST66 being the most common and widespread clones. A ST78 isolate was positive for KPC-2, and a ST114 isolate was positive for VIM-1 (18). With the exception of this study from Izedebski et al (18), limited information is available regarding the global distribution of ST93, ST90, ST78, ST105, and ST108 and consists mainly of sporadic reports (29–32).
Chavda et al. (9) characterized 74 carbapenem-resistant Enterobacter spp. (more than half of the isolates were obtained from New Jersey, USA), and most possessed different blaKPCs, whereas only 2 isolates had blaNDM-1. E. xiangfangensis also dominated, and ST171 was the most common clone. ST171 was rare in our collection (n = 4) but did show genetic and geographic diversity. ST171 was divided into 3 clades: 171A, 171B, and 171C (Technical Appendix 2 Figure). Clades 171B and 171C are associated with blaKPC from the United States and United Kingdom (Technical Appendix 2 Figure). Clade 171B (n = 2) contained blaKPC-2 from Colombia and blaNDM-1 from Guatemala. Clade 171A (n = 1) with blaNDM-1 was obtained from South Africa, and clade 171C with blaKPC-3 was obtained from the United States.
We noted interesting associations and geographic distribution between genetic structures surrounding carbapenemase genes and clades, clones, and species. First, identical genetic structures were situated in various STs within the same or different institutions of the same country (e.g., NDM-GE01 with blaNDM-1 in Vietnam; In87 and In237 with blaVIM-1 in Greece; In916 with blaVIM-1 in Italy; In624 with blaVIM-1 in Spain; and NDM-GE03 with blaNDM-1 in Guatemala). Second, identical genetic structure was present in different STs (ST105 and ST520), from different countries (e.g., In110 with blaVIM-1 in Croatia and Spain). Third, different genetic structures were present in the same STs and clades obtained from different countries (e.g., ST78 with In237 from Greece, ST78 with In916 from Italy, ST78 with In624 from Spain, ST114A with NDM-GE02 from Serbia, and ST114A with pNDM-U.S. from Romania). Last, an identical genetic structure (NDM-GE-U.S.) was found in different global species, STs, and clades.
These associations demonstrate that certain mobile genetic elements with carbapenemase genes have the ability to move between clones and clades of Enterobacter spp. on a global scale. This ability is highlighted by ST78 with blaVIM-1 within different integrons (In237, In916, and In624) that circulate between various countries (Greece, Italy, and Spain). As some STs are introduced into different countries, they apparently acquire the local genetic elements prevalent in that country. Of special concern is the description of a common NDM genetic structure, named NDM-GE-U.S., previously found on pNDM-U.S. and first described in a K. pneumoniae from the United States (24). NDM-GE-U.S. was present in different species, clones, and clades obtained from 6 countries spanning 4 continents. Sequence similarity analysis suggested that it was present on different types of plasmids (pK518_NDM1 and pNDM-HN380) among Enterobacter spp. with blaNDM.
Our results support the current understanding that the carbapenem resistance pandemic is the consequence of circulating clones and the spread of mobile genetic elements. We found that certain clones and clades (ST78, ST90C, ST96, ST114A, ST114C, and ST141) containing particular genetic structures (In87, In624, In916, In237, NDM-GE01, NDM-GE02, and NDM-GE03) and carbapenemases were circulating locally within the same or between different institutions in certain countries (Greece, Guatemala, Italy, Spain, Serbia, and Vietnam). Other global clones and clades (ST90B, ST93, and ST108) contained various genetic structures and carbapenemases.
A limitation of this study was that plasmids harboring carbapenemases were not reconstructed because of the limitations of short-read sequencing (33). The characterization of plasmids is vital to fully comprehend the molecular epidemiology of Enterobacter spp. with carbapenemases, and a follow-up study using long-read sequencing is currently under way. In the meantime, our study highlights the importance of surveillance programs using whole-genome sequencing to provide insight into the characteristics and global distribution of clones and clades as well as their association with mobile genetic elements surrounding the different carbapenemase genes.
Dr. Peirano is a research associate at Calgary Laboratory Services and the University of Calgary. Her main research interests revolve around the detection and molecular epidemiology of antimicrobial drug resistance mechanisms among gram-negative bacteria.
Acknowledgments
This work was supported by the John Mung Program from Kyoto University, Japan (Y.M.) and a research grant from the Calgary Laboratory Services (grant no. 10015169) to J.D.D.P. This work was also supported in part by National Institutes of Health grant nos. R01AI090155 (B.N.K.) and R21AI117338 (L.C.), and the Genome Center for Infectious Diseases grant no. U19AI110819 from the National Institutes of Health’s National Institute of Allergy and Infectious Diseases (J.C.V.I.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organizations had no role in study design, data collection and interpretation, or the decision to submit the work for publication
Transparency declarations: J.D.D.P. had previously received research funds from Merck and AstraZeneca. P.B. is an employee of AstraZeneca, and M.M. is an employee of Merck. All other authors have nothing to declare.
References
- Jean SS, Hsueh PR; SMART Asia-Pacific Group. Distribution of ESBLs, AmpC β-lactamases and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal and urinary tract infections in the Asia-Pacific region during 2008-14: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). J Antimicrob Chemother. 2017;72:166–71. DOIPubMed
- Kanamori H, Parobek CM, Juliano JJ, van Duin D, Cairns BA, Weber DJ, et al. A prolonged outbreak of KPC-3-producing Enterobacter cloacae and Klebsiella pneumoniae driven by multiple mechanisms of resistance transmission at a large academic burn center. Antimicrob Agents Chemother. 2017;61:e01516–16.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27919898&dopt=Abstract
- US Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:750.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20577157&dopt=Abstract
- Hoffmann H, Stindl S, Ludwig W, Stumpf A, Mehlen A, Heesemann J, et al. Reassignment of enterobacter dissolvens to Enterobacter cloacae as E. cloacae subspecies dissolvens comb. nov. and emended description of Enterobacter asburiae and Enterobacter kobei. Syst Appl Microbiol. 2005;28:196–205. DOIPubMed
- Gu CT, Li CY, Yang LJ, Huo GC. Enterobacter xiangfangensis sp. nov., isolated from Chinese traditional sourdough, and reclassification of Enterobacter sacchari Zhu et al. 2013 as Kosakonia sacchari comb. nov. Int J Syst Evol Microbiol. 2014;64:2650–6.
- Arredondo-Alonso S, Willems RJ, van Schaik W, Schürch AC. On the (im)possibility of reconstructing plasmids from whole-genome short-read sequencing data. Microb Genom. 2017;3:e000128.
Figures
Cite This ArticleOriginal Publication Date: 5/4/2018
1These co-authors contributed equally to this article.
2Current affiliation: Jackson Laboratory for Genomic Medicine, Farmington, Connecticut, USA.


































No hay comentarios:
Publicar un comentario