
Trastuzumab May Improve Survival in Women with Rare Endometrial Cancer
, by NCI Staff
Some women with an aggressive type of endometrial cancer may live longer if they receive a drug that is commonly used to treat a form of breast cancer, according to updated results from a small clinical trial.
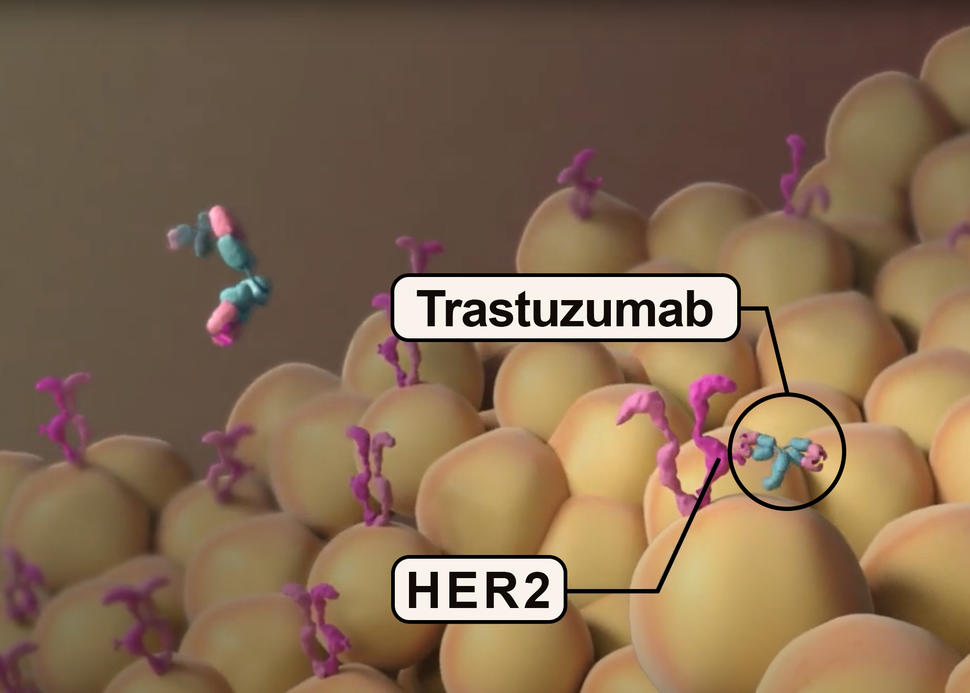
Women in the trial had uterine serous carcinoma (USC) that had either spread beyond the uterus or returned after previous treatment and had high levels of HER2, a protein that can fuel the growth of cancer cells. Trastuzumab (Herceptin) binds to HER2 on the surface of cancer cells and blocks it from making the tumor grow. The drug has been used to treat breast cancer with high HER2 levels since 1998.
“The results from this trial should alert doctors and patients to the importance of HER2 in endometrial cancer,” said Elise Kohn, M.D., gynecologic cancers lead for NCI’s Division of Cancer Treatment and Diagnosis.
The updated survival results were published June 29 in Clinical Cancer Research. The trial was sponsored by Yale University and Genentech, the maker of trastuzumab.
A Cancer Type on the Rise
Endometrial cancer is the most common gynecologic cancer in the United States, with an estimated 65,620 new cases in 2020. And the incidence of endometrial cancer has been on the rise, with the number of cases doubling over the last 20 years.
The most common type of the disease, called endometrioid endometrial carcinoma, is usually diagnosed at an early stage and can often be cured with an operation called a hysterectomy to remove the uterus, sometimes followed by radiation therapy.
Uterine serous carcinoma is an aggressive type of endometrial cancer. Although it accounts for only 10% to 15% of endometrial cancer cases, USC is responsible for about 40% of deaths from the disease. Unlike the more common endometroid endometrial carcinoma, USC tends to be diagnosed at a more advanced stage.
“When we detect these tumors, they have usually already spread, either to the lymph nodes or to other parts of the body,” said the study’s lead investigator Alessandro Santin, M.D., professor of Obstetrics, Gynecology, and Reproductive Sciences at the Yale Cancer Center. That is why, to treat USC, you need a body-wide, or systemic, treatment, in addition to surgery, that can effectively control the spread of the disease outside of the uterus, he explained.
In about one-third of women with USC, their tumor cells overproduce HER2, which has been shown to indicate poor prognosis in women with endometrial cancer. In addition, Black women with endometrial cancer are more likely than White women to be diagnosed with UCS. Studies, including one led by Dr. Santin, have suggested that Black women with USC are more likely than women of other ethnicities/races to have HER2-positive tumors. About one-third of the women treated in this recent clinical trial were Black.
Trial Results Point to Potential Benefits
This clinical trial included 58 women with HER2-positive USC that had spread beyond the uterus or returned after treatment who had undergone a hysterectomy. They were assigned by chance to receive standard treatment with the chemotherapy drugs carboplatin and paclitaxel or the same treatment plus trastuzumab.
The primary goal of the study was to determine how long the women lived during and after treatment before their cancer started growing again (progression-free survival). This was longer for women who received trastuzumab plus standard chemotherapy than for women who received standard chemotherapy alone (13 months versus 8 months). Women who received trastuzumab also lived longer overall than women who received chemotherapy alone (29.6 months versus 24.4 months).
Almost all women in the study experienced side effects, but no participants stopped treatment because of them. The types of side effects did not differ significantly between the two treatment groups, however, women receiving trastuzumab were more likely to experience high blood pressure.
The researchers noted that women with advanced cancer—that is, cancer that had spread beyond the uterus—who had not received prior systemic therapy benefited more from the addition of trastuzumab than women whose cancer had returned following prior systemic treatment. After 26 months of follow-up, more than half of the women who received trastuzumab plus chemotherapy as their first systemic treatment were still alive. Those women who received only chemotherapy for their initial treatment lived about 24.4 months.
Progression-free survival was longer for previously untreated women receiving trastuzumab as well, 17.7 months compared with 9.3 months for women receiving chemotherapy alone.
“We have been able to show for the first time that adding the HER2-targeted agent trastuzumab to the standard chemotherapy regimen may make a huge difference, not only in terms of progression-free survival, but also in terms of overall survival. These women live significantly longer,” Dr. Santin explained.
Small Study, Provocative Findings
The study was launched in 2011 and was designed to enroll 100 women with HER2-positive USC. But after 6 years of recruitment, only 61 women had enrolled, and the study stopped accepting new participants. Of the women who had enrolled, 58 received treatment and were included in the analysis.
Despite its small size and lower than anticipated number of patients, the study’s initial results—published in 2018—led the National Comprehensive Cancer Network, an independent network of cancer centers that develops clinical practice guidelines, to include the use of trastuzumab in its treatment recommendations for women with this form of USC.
“Even with 58 patients divided into two [treatment groups], we were able to see a difference for both progression-free survival and overall survival,” Dr. Santin said. “These results tell a lot about how strongly HER2 drives this cancer, meaning treatment with trastuzumab in this patient population can make a difference.”
Although the study showed a benefit to patients, these results need to be confirmed by a larger study, said Dr. Kohn.
“This really was a small study, with a mixed population of women with [previously untreated] as well as recurrent [USC],” Dr. Kohn explained. “But within those confines, it was positive, and the benefit in overall survival, despite not being the primary endpoint, was certainly provocative.”
A larger phase 3 clinical trial is being planned to confirm the phase 2 results.
HER2 Testing Is Key
Some academic medical centers test for high HER2 levels in endometrial cancer, according to both Drs. Santin and Kohn, but the extent to which such tumor testing is done by community cancer centers and private practices isn’t known.
“We consider [laboratory testing for HER2 in USC] standard of care [at Yale],” Dr. Santin said.
Dr. Kohn thinks there’s room for improvement when it comes to molecular testing in women with USC. “It's done typically in some of the academic centers, but I don't think it is done broadly,” she said.
Uterine serous carcinoma is “a small but severe subset of endometrial cancer” that frequently displays high levels of HER2, and the preliminary data from the study strongly suggest that all women diagnosed with USC should be tested, said Dr. Kohn.
In breast cancer and gastric cancer, HER2 is a negative prognostic biomarker, “meaning patients with [high HER2 levels on their tumors] usually have worse outcomes,” Dr. Kohn said. “But in breast cancer and gastric cancer, it's also a positive predictive sign, meaning that if you treat the cancer with a HER2-targeted regimen, you may be able to [improve how long patients live].”
This study suggests the same may be true for this aggressive subtype of endometrial cancer.
“So that's an important step forward that we want to confirm,” she said.
Dr. Santin explained that doctors look at several proteins on the tumor to diagnose USC, but none of those proteins change how the cancer is treated.
Adding a HER2 test to the diagnostic workup may have a huge impact for the patient because it may open up a new treatment that could extend their overall survival, he concluded.





















.png)












No hay comentarios:
Publicar un comentario