
Scientists Focus on Fusion Proteins in Childhood Cancers
, by NCI Staff
Cancer researchers have teamed up with experts in fields such as chemistry and structural biology to study a hallmark of childhood cancers: fusion proteins.
Also known as fusion oncoproteins, these abnormal molecules are made of the fused parts of two proteins. Although fusion oncoproteins may drive the development of many cancers in children, researchers still know little about the biology of most of these diseases.
To learn more, NCI launched the Fusion Oncoproteins in Childhood Cancers (FusOnC2) Consortium. The group includes researchers who have expertise in such disciplines as cancer biology, proteomics, genomics, computational biology, and pharmacology.
“The goal of the consortium is to bring together experts who have different skills and perspectives to think about fusion proteins in new ways,” said Keren Witkin, Ph.D., of NCI’s Division of Cancer Biology, who coordinates the group.
“What’s different about the FusOnC2 Consortium,” Dr. Witkin continued, “is that we are really trying to push the team science approach. We want to get the molecular biologists working with the medicinal chemists and the computational biologists.”
Researchers participating in the consortium—which is part of the Cancer Moonshot℠ initiative to accelerate progress in cancer research—are investigating various cancers driven by fusion proteins in children and young adults. Their focus is difficult-to-treat cancers.
For example, some of the researchers are studying synovial sarcoma and rhabdomyosarcoma, which develop in soft tissue, and ependymoma, a brain cancer.
“What the investigators learn could guide future research on treatments for patients with fusion protein–driven cancers,” Dr. Witkin said. “The consortium includes a number of clinical oncologists and two patient advocates. We’re doing this to find cures for patients.”
Challenges in Targeting Fusion Proteins
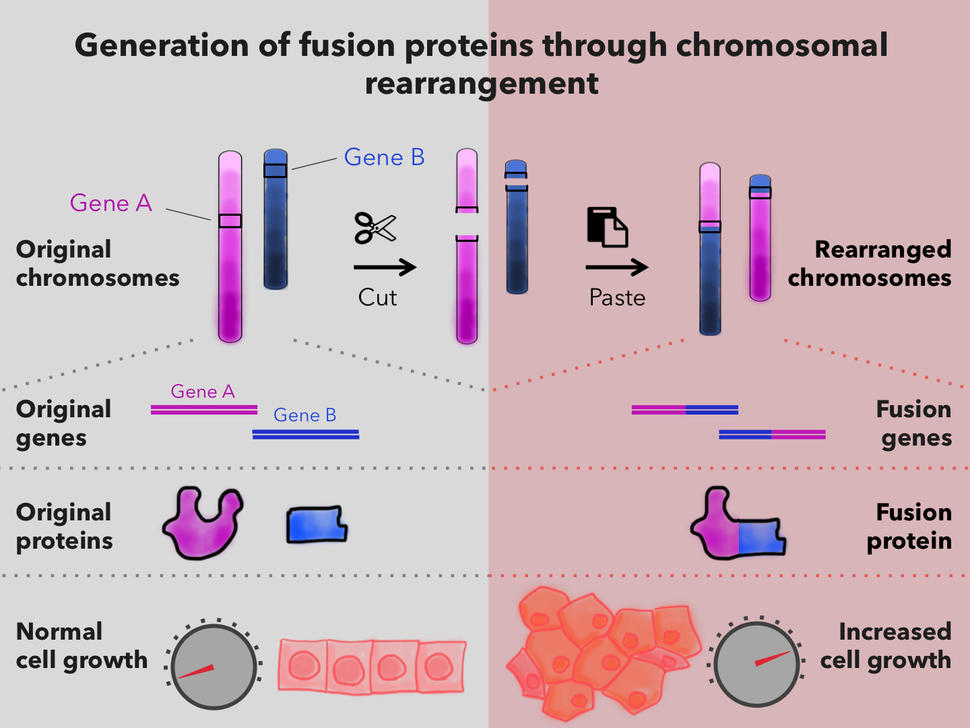
Fusion proteins occur in cells in which a piece of one chromosome breaks off and attaches to another chromosome. When DNA segments from different chromosomes fuse, the result can be an entirely new gene—a fusion gene.
Not all fusion genes lead to cancer. But in some cells, a fusion gene produces fusion proteins that can alter the activity of other genes. In some cases, the altered gene activity causes those cells to grow uncontrollably and form tumors.
“Chromosome breaks leading to fusion oncoproteins are very common in childhood cancers,” said Kimberly Stegmaier, M.D., of the Dana-Farber Cancer Institute, who is leading one of the research teams.
“We can identify them in many leukemias, solid tumors, and brain cancers in children,” she added, “and they are often a key initiating event in the cancer.”
In theory, Dr. Witkin said, “fusion oncoproteins are terrific drug targets, because they drive the disease and are present only in cancer cells.” Drugs that target tumor cells while sparing healthy cells may reduce the risk of side effects.
“But in practice,” Dr. Witkin continued, “it has been very difficult to find drugs that target only fusion oncoproteins.”
Several drugs that are effective against fusion oncoproteins have been developed, such as imatinib (Gleevec), which blocks the activity of the BCR-ABL fusion protein. This fusion protein drives nearly all cases of chronic myelogenous leukemia as well as some other types of leukemia.
Part of the BCR-ABL fusion protein is a kinase, which is a type of enzyme, and drugs that target kinase fusion proteins have generally worked well. Some patients have had dramatic and long-lasting responses to these drugs, noted Dr. Stegmaier.
Many fusion oncoproteins in children, however, involve transcription factors, which are proteins that control the activity of genes by binding to DNA. Efforts to target these oncoproteins have been largely unsuccessful. Unlike kinases, transcription factors tend not to have “pockets” where a drug could enter, as a key enters a lock.
“For the pediatric cancer field, the holy grail has been to find a way to directly target the fusions that involve transcription factors,” said Dr. Stegmaier.
Dr. Witkin agreed. “If we could figure out how to target transcription factors in fusion oncoproteins, it would be a tremendous breakthrough.”
Two Main Research Strategies
From the start, the consortium has emphasized collaboration. Last year, for example, the investigators met to exchange ideas and to consider next steps in the research.
“The goal of the consortium is to bring together people who may be experts on an individual fusion oncoprotein and encourage them to think collectively about fusion-driven cancers in children,” said Cigall Kadoch, Ph.D., also of the Dana-Farber Cancer Institute, and another lead investigator.
The research, Dr. Kadoch continued, may reveal biological insights that are relevant across multiple diseases.
Dr. Witkin described two main research strategies for developing new therapies for these cancers. “One is to target fusion oncoproteins directly,” she said. “This is the most straightforward approach, and people have tried it without great success.”
The other strategy is to target proteins that interact with the fusion protein or that play an important role in the growth and survival of cancer cells that have the fusion protein.
“If an oncoprotein cannot be targeted directly, perhaps we can target another step in a pathway involved in driving the cancer,” Dr. Witkin explained. “By developing a better understanding of the molecular changes associated with oncoproteins, we might be able to exploit this information to develop therapies.”
Degrading Fusion Oncoproteins
Dr. Stegmaier and her colleagues are applying these approaches to Ewing sarcoma, a type of solid tumor that forms in bone or soft tissue. The cancer occurs most often in teenagers and young adults.
For many patients, a gene fusion that leads to the fusion protein EWS-FLI1 is the key event that initiates the disease. As with other fusion-driven childhood cancers, Ewing sarcoma cells usually have few other genetic mutations beyond the fusion itself.
Treatments for Ewing sarcoma that has not overtly spread within the body have improved in recent years, but there have not been similar advances for patients with metastatic disease. “We really need better therapies,” said Dr. Stegmaier.
“Ewing sarcoma has not been highly responsive to the types of targeted therapies that have worked in other cancers, and, in general, immunotherapy has not worked,” she continued. “So, researchers always come back to the fusion.”
One approach she and her colleagues have been exploring is to try to degrade the fusion oncoprotein, essentially removing it from cells. This approach has worked in another fusion protein–driven cancer called acute promyelocytic leukemia (APL) and has increased long-term survival for patients dramatically, noted Dr. Stegmaier. A combination of therapies has contributed to the improved results. Treatment options for patients with APL include therapies such as all-trans retinoic acid and arsenic trioxide, which lead to degradation of the APL fusion, as well as chemotherapy.
“The APL example demonstrates that if you can find a way to effectively get rid of the fusion, you might be able to cure the disease,” Dr. Stegmaier explained. “APL is a beautiful example of how figuring out how to effectively target the fusion has been tremendously successful.”
“This idea of degradation is powerful, because making the fusion protein go away fully could be more effective than just inhibiting its activity,” Dr. Stegmaier continued. “People are excited about this concept, and it’s one of the major ideas in our projects.”
However, she went on, “we’re trying to take a multipronged approach—including evaluating the complexes that EWS-FLI1 forms—because we know that any one of the strategies will have its challenges.”
How a Fusion Protein Hijacks a Protein Complex
Dr. Kadoch’s lab focuses on the regulation of genes and the physical structures of DNA in human development and in cancer. Her FusOnC2 team is investigating synovial sarcoma, a rare soft tissue sarcoma that primarily affects adolescents and young adults.
All cases of synovial sarcoma are driven by a fusion oncoprotein called SS18-SSX. In previous studies, Dr. Kadoch and her colleagues have demonstrated that the fusion oncoprotein causes the activation, or expression, of certain genes that are not normally expressed in cells without the fusion.
The fusion oncoprotein does this by “hijacking” a collection, or complex, of proteins in the cell nucleus—known as the BAF complex—that switches genes on and off. “The fusion protein takes BAF complexes away from genes that it is normally supposed to turn on and brings it to genes that should not be activated,” said Dr. Kadoch.
Some of the improperly activated genes are involved in the maintenance of stem cell pathways that help support the growth of synovial sarcoma cells, she noted. “Essentially, the fusion oncoprotein sets up a whole new program of gene expression in cells by bringing BAF complexes to the wrong sites at the wrong times.”
Although researchers have known that the BAF complex activates genes by altering the architecture of DNA, the mechanisms by which the SS18-SSX fusion protein disrupts this process remain poorly understood. The FusOnC2 team aims to reveal the molecular mechanisms responsible.
“If we know the mechanisms, then we could take that information and explore ways to prevent the changes to gene expression,” Dr. Kadoch said.
Fusion Proteins and Leukemia
Another member of the consortium, Charles Mullighan, M.D., of St. Jude Children’s Research Hospital, is investigating a family of fusion proteins involving NUP98. Up to 10% of cases of acute myeloid leukemia (AML) in children have fusion proteins involving NUP98, and patients with these cancers have a poor prognosis. There are no effective treatments.
The researchers are building a collection of complementary research tools, including mouse models that closely resemble how AML develops in humans, to study how NUP98 fusion proteins contribute to cancer.
“We wanted to go really broad on this project and not just use conventional approaches,” Dr. Mullighan said. His team will use CRISPR genome-editing tools to search for molecules that are essential for the survival of leukemia cells with NUP98 fusion proteins and that could represent potential targets. They will also explore strategies for directly degrading NUP98 fusion proteins.
In addition, the investigators will analyze the role of NUP98 fusion proteins in a process known as liquid‒liquid phase separation, which is a way that cells organize certain proteins without enclosing them in a membrane.
Researchers have hypothesized that the formation of these membrane-less clusters of proteins may be another way that cells regulate the expression of genes.
There is evidence from imaging studies that some NUP98 fusion proteins contribute to the abnormal formation of protein clusters, noted Dr. Mullighan. “We know there are key parts of the NUP98 fusion protein that are important in driving phase separation,” he said.
His team, which includes Richard W. Kriwacki, Ph.D., a structural biologist at St. Jude who studies phase separation, will ask whether phase separation is needed to drive the changes in gene expression that may be important in promoting leukemia.
“Phase separation has become a big part of the fusion oncoprotein field, and it’s poorly understood,” said Dr. Witkin. “But there seems to be agreement among researchers that phase separation seems to drive high levels of gene activity.”
“We think that phase separation may be involved in turning on genes involved in embryonic development that should have been turned off after a child is born,” she added. “This is a common theme of childhood cancers.”
New Opportunities for Investigating Fusion Proteins
The FusOnC2 Consortium aims to build on recent advances in research. “Many of us have become quite excited over the last several years because of new ideas in chemistry and other areas,” said Dr. Stegmaier. “Each of our projects has been enabled by improvements in technology.”
For example, improvements in technologies for analyzing proteins, including mass spectrometry and cryo-electron microscopy (cryo-EM), have enhanced the research community’s ability to identify and study protein complexes that play a role in childhood cancers, she noted.
At the same time, genomic studies have revealed the range of genetic alterations in childhood cancers, noted Dr. Mullighan.
“The biology of pediatric fusion oncoproteins in tumors has been studied for decades,” he said. “But now, for the first time, we can investigate these diseases systematically using a great deal of genomic information plus new experimental tools and new therapeutic approaches.”






















.png)












No hay comentarios:
Publicar un comentario