
Duration of Isolation and Precautions for Adults with COVID-19
Note:
At this time, we do not know if someone can be re-infected with COVID-19. Data to date show that a person who has had and recovered from COVID-19 may have low levels of virus in their bodies for up to 3 months after diagnosis. This means that if the person who has recovered from COVID-19 is retested within 3 months of initial infection, they may continue to have a positive test result, even though they are not spreading COVID-19.
There are no confirmed reports to date of a person being reinfected with COVID-19 within 3 months of initial infection. However, additional research is ongoing. Therefore, if a person who has recovered from COVID-19 has new symptoms of COVID-19, the person may need an evaluation for reinfection, especially if the person has had close contact with someone infected with COVID-19. The person should isolate and contact a healthcare provider to be evaluated for other causes of their symptoms, and possibly retested.
Until we know more, CDC recommends that all people, whether or not they have had COVID-19, continue to take safety measures to avoid becoming infected with COVID-19 (wash hands regularly, stay at least 6 feet away from others whenever possible, and wear masks).
For more information:
On This Page
Accumulating evidence supports ending isolation and precautions for persons with COVID-19 using a symptom-based strategy. This update incorporates recent evidence to inform the duration of isolation and precautions recommended to prevent transmission of SARS-CoV-2 to others, while limiting unnecessary prolonged isolation and unnecessary use of laboratory testing resources.
Key findings are summarized here.
- Concentrations of SARS-CoV-2 RNA measured in upper respiratory specimens decline after onset of symptoms (CDC, unpublished data, 2020; Midgley et al., 2020; Young et al., 2020; Zou et al., 2020; Wölfel et al., 2020; van Kampen et al., 2020).
- The likelihood of recovering replication-competent virus also declines after onset of symptoms. For patients with mild to moderate COVID-19, replication-competent virus has not been recovered after 10 days following symptom onset (CDC, unpublished data, 2020; Wölfel et al., 2020; Arons et al., 2020; Bullard et al., 2020; Lu et al., 2020; personal communication with Young et al., 2020; Korea CDC, 2020). Recovery of replication-competent virus between 10 and 20 days after symptom onset has been documented in some persons with severe COVID-19 that, in some cases, was complicated by immunocompromised state (van Kampen et al., 2020). However, in this series of patients, it was estimated that 88% and 95% of their specimens no longer yielded replication-competent virus after 10 and 15 days, respectively, following symptom onset.
- A large contact tracing study demonstrated that high-risk household and hospital contacts did not develop infection if their exposure to a case patient started 6 days or more after the case patient’s illness onset (Cheng et al., 2020).
- Although replication-competent virus was not isolated 3 weeks after symptom onset, recovered patients can continue to have SARS-CoV-2 RNA detected in their upper respiratory specimens for up to 12 weeks (Korea CDC, 2020; Li et al., 2020; Xiao et al, 2020). Investigation of 285 “persistently positive” persons, which included 126 persons who had developed recurrent symptoms, found no secondary infections among 790 contacts attributable to contact with these case patients. Efforts to isolate replication-competent virus from 108 of these case patients were unsuccessful (Korea CDC, 2020).
- Specimens from patients who recovered from an initial COVID-19 illness and subsequently developed new symptoms and retested positive by RT-PCR did not have replication-competent virus detected (Korea CDC, 2020; Lu et al., 2020). The risk of reinfection may be lower in the first 3 months after initial infection, based on limited evidence from another betacoronavirus (HCoV-OC43), the genus to which SARS-CoV-2 belongs (Kiyuka et al, 2018).
- Currently, 6 months after the emergence of SARS-CoV-2, there have been no confirmed cases of SARS-CoV-2 reinfection. However, the number of areas where sustained infection pressure has been maintained, and therefore reinfections would be most likely observed, remains limited.
- Serologic or other correlates of immunity have not yet been established.
The current evidence includes the following caveats:
- In a recent study of skilled nursing facility workers followed prospectively for asymptomatic infection, one of 48 infected staff had a nasopharyngeal swab which was weakly positive on a single-passage plaque assay more than 20 days after initial diagnosis; however, the specimen was not subjected to serial passage to demonstrate the presence of replication-competent virus (Quicke et al., 2020).
- In one case report, a person with mild illness provided specimens that yielded replication-competent virus for up to 18 days after symptom onset (Liu et al., 2020).
- Data currently available are derived from adults; equivalent data from children and infants are not presently available.
- More data are needed concerning viral shedding in some situations, including in immunocompromised persons.
Assessment
Available data indicate that persons with mild to moderate COVID-19 remain infectious no longer than 10 days after symptom onset. Persons with more severe to critical illness or severe immunocompromise likely remain infectious no longer than 20 days after symptom onset. Recovered persons can continue to shed detectable SARS-CoV-2 RNA in upper respiratory specimens for up to 3 months after illness onset, albeit at concentrations considerably lower than during illness, in ranges where replication-competent virus has not been reliably recovered and infectiousness is unlikely. The etiology of this persistently detectable SARS-CoV-2 RNA has yet to be determined. Studies have not found evidence that clinically recovered persons with persistence of viral RNA have transmitted SARS-CoV-2 to others. These findings strengthen the justification for relying on a symptom based, rather than test-based strategy for ending isolation of these patients, so that persons who are by current evidence no longer infectious are not kept unnecessarily isolated and excluded from work or other responsibilities.
Reinfection with SARS-CoV-2 has not yet been definitively confirmed in any recovered persons to date. If, and if so when, persons can be reinfected with SARS-CoV-2 remains unknown and is a subject of investigation. Persons infected with related endemic human betacoronavirus appear to become susceptible again at around 90 days after onset of infection. Thus, for persons recovered from SARS-CoV-2 infection, a positive PCR during the 90 days after illness onset more likely represents persistent shedding of viral RNA than reinfection.
- If such a person remains asymptomatic during this 90-day period, then any re-testing is unlikely to yield useful information, even if the person had close contact with an infected person.
- If such a person becomes symptomatic during this 90-day period and an evaluation fails to identify a diagnosis other than SARS-CoV-2 infection (e.g., influenza), then the person may warrant evaluation for SARS-CoV-2 reinfection in consultation with an infectious disease or infection control expert. Isolation may be warranted during this evaluation, particularly if symptoms developed after close contact with an infected person.
Correlates of immunity to SARS-CoV-2 infection have not been established. Specifically, the utility of serologic testing to establish the absence or presence of infection or reinfection remains undefined.
The recommendations below are based on the best information available in mid-July 2020 and reflect the realities of an evolving pandemic. Even for pathogens for which many years of data are available, it may not be possible to establish recommendations that ensure 100% of persons who are shedding replication-competent virus remain isolated. CDC will continue to closely monitor the evolving science for information that would warrant reconsideration of these recommendations.
Recommendations
- Duration of isolation and precautions
- For most persons with COVID-19 illness, isolation and precautions can generally be discontinued 10 days after symptom onset1 and resolution of fever for at least 24 hours, without the use of fever-reducing medications, and with improvement of other symptoms.
- A limited number of persons with severe illness may produce replication-competent virus beyond 10 days that may warrant extending duration of isolation and precautions for up to 20 days after symptom onset; consider consultation with infection control experts.
- For persons who never develop symptoms, isolation and other precautions can be discontinued 10 days after the date of their first positive RT-PCR test for SARS-CoV-2 RNA.
- For most persons with COVID-19 illness, isolation and precautions can generally be discontinued 10 days after symptom onset1 and resolution of fever for at least 24 hours, without the use of fever-reducing medications, and with improvement of other symptoms.
- Role of PCR testing2 to discontinue isolation or precautions
- For persons who are severely immunocompromised, a test-based strategy could be considered in consultation with infectious diseases experts.
- For all others, a test-based strategy is no longer recommended except to discontinue isolation or precautions earlier than would occur under the strategy outlined in Part 1, above.
- Role of PCR testing2 after discontinuation of isolation or precautions
- For persons previously diagnosed with symptomatic COVID-19 who remain asymptomatic after recovery, retesting is not recommended within 3 months after the date of symptom onset for the initial COVID-19 infection.
- For persons who develop new symptoms consistent with COVID-19 during the 3 months after the date of initial symptom onset, if an alternative etiology cannot be identified by a provider, then the person may warrant retesting; consultation with infectious disease or infection control experts is recommended. Isolation may be considered during this evaluation based on consultation with an infection control expert, especially in the event symptoms develop within 14 days after close contact with an infected person.
- For persons who never developed symptoms, the date of first positive RT-PCR test for SARS-CoV-2 RNA should be used in place of the date of symptom onset.
- Role of serologic testing
- Serologic testing should not be used to establish the presence or absence of SARS-CoV-2 infection or reinfection.
[1] Symptom onset is defined as the date on which symptoms first began, including non-respiratory symptoms.
[2] PCR testing is defined as the use of an RT-PCR assay to detect the presence of SARS-CoV-2 RNA..
[2] PCR testing is defined as the use of an RT-PCR assay to detect the presence of SARS-CoV-2 RNA..
References
- Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020 May 28;382(22):2081-2090. doi:10.1056/NEJMoa2008457.
- Bullard J, Durst K, Funk D, Strong JE, Alexander D, Garnett L et al. Predicting Infectious SARS-CoV-2 From Diagnostic Samples. Clin Infect Dis 2020 May 22. doi: 10.1093/cid/ciaa638.
- Cheng HW, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH, et al. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern Med 2020 May 1; doi:10.1001/jamainternmed.2020.2020.
- Kiyuka PK, Agoti CN, Munywoki PK, Njeru R, Bett A, Otieno JR, et al. Human Coronavirus NL63 Molecular Epidemiology and Evolutionary Patterns in Rural Coastal Kenya. J Infect Dis 2018 May 5;217(11):1728-1739. doi: 10.1093/infdis/jiy098.
- Korea Centers for Disease Control and Prevention. Findings from Investigation and Analysis of re-positive cases. May 19, 2020. Available at: https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=367267&nPage=1
- Li N, Wang X, Lv T. Prolonged SARS-CoV-2 RNA Shedding: Not a Rare Phenomenon. J Med Virol 2020 Apr 29. doi: 10.1002/jmv.25952.
- Liu WD, Chang SY, Wang JT, Tsai MJ, Hung CC, Hsu CL, et al. Prolonged Virus Shedding Even After Seroconversion in a Patient With COVID-19. J Infect 2020 Apr 10;S0163-4453(20)30190-0. doi: 10.1016/j.jinf.2020.03.063
- Lu J, Peng J, Xiong Q, Liu Z, Lin H, Tan X, et al. Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR. (Preprint) Medrxiv. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.06.15.20131748v1 doi: https://doi.org/10.1101/2020.06.15.20131748
- Midgley CM, Kujawski SA, Wong KK, Collins, JP, Epstein L, Killerby ME et al. (2020). Clinical and Virologic Characteristics of the First 12 Patients with Coronavirus Disease 2019 (COVID-19) in the United States. Nat Med 2020 Jun;26(6):861-868. doi: 10.1038/s41591-020-0877-5.
- Quicke K, Gallichote E, Sexton N, Young M, Janich A, Gahm G, et al. Longitudinal Surveillance for SARS-CoV-2 RNA Among Asymptomatic Staff in Five Colorado Skilled Nursing Facilities: Epidemiologic, Virologic and Sequence Analysis. (Preprint) Medrxiv. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.06.08.20125989v1 doi: https://doi.org/10.1101/2020.06.08.20125989
- van Kampen J, van de Vijver D, Fraaij P, Haagmans B, Lamers M, Okba N, et al. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. (Preprint) Medrxiv. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.06.08.20125310v1 doi: https://doi.org/10.1101/2020.06.08.20125310
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. (2020). Virological assessment of hospitalized patients with COVID-2019. Nature 2020 May;581(7809):465-469. doi:10.1038/s41586-020-2196-x
- Xiao F, Sun J, Xu Y, Li F, Huang X, Li H, et al. Infectious SARS-CoV-2 in Feces of Patient with Severe COVID-19. Emerg Infect Dis 2020;26(8):10.3201/eid2608.200681. doi:10.3201/eid2608.200681
- Young BE, Ong SWX, Kalimuddin S, Low JG, Ta, SY, Loh J, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020 Mar 3;323(15):1488-1494. doi:10.1001/jama.2020.3204
- Personal communication with Young BE first author of preprint of: Young BE, Ong SW, Ng LF, Anderson DE, Chia WN, Chia PY, et al. Immunological and Viral Correlates of COVID-19 Disease Severity: A Prospective Cohort Study of the First 100 Patients in Singapore. (Preprint) SSRN. 2020. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3576846 http://dx.doi.org/10.2139/ssrn.3576846
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. (2020). SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med, 382(12), 1177-1179. doi:10.1056/NEJMc200173
Figure 1: From Wölfel et al., demonstrating declining viral burden in upper respiratory specimens as illness progresses and decreasing capacity to isolate replication-competent virus from these same specimens as the number of patients with detectable IgM and IgG increases.
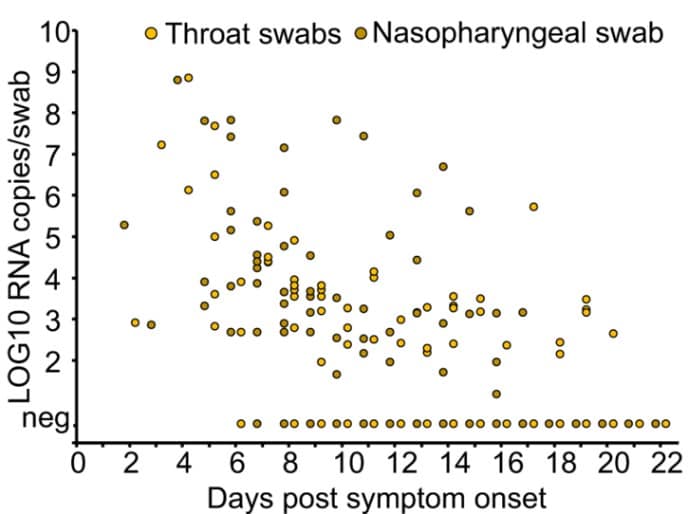
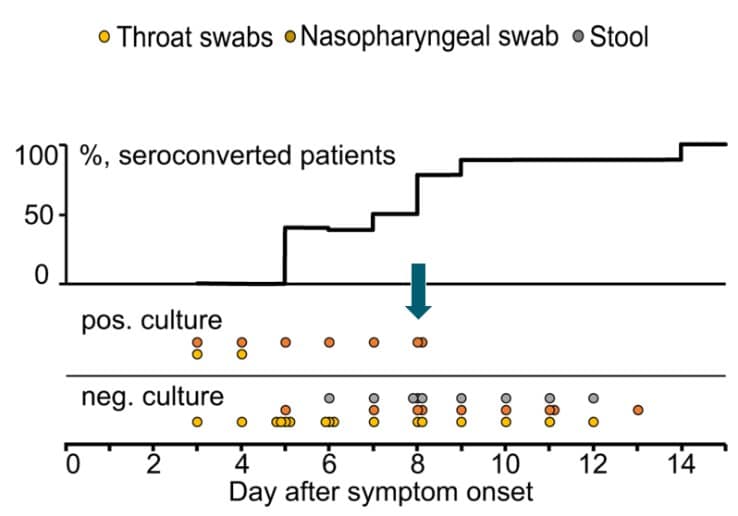
Figure 2: From Midgley et al., demonstrating inability to recover replication-competent virus from specimens collected more than 9 days after illness onset. Kaplan-Meier analysis shows time to inability to recover replication-competent SARS-CoV-2 from 14 U.S. patients. Last probability of successful isolation falls to 50% at day 4 after illness onset and to 80% at day 8. After day 9, probability approaches zero. Unpublished CDC data.
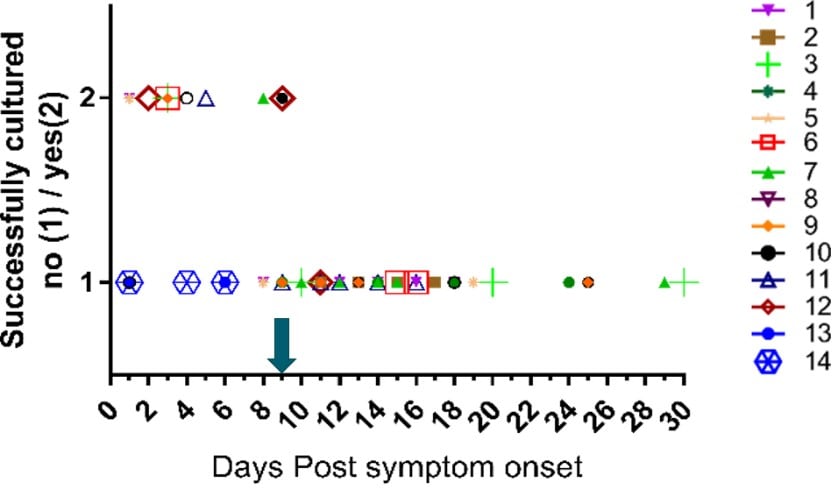
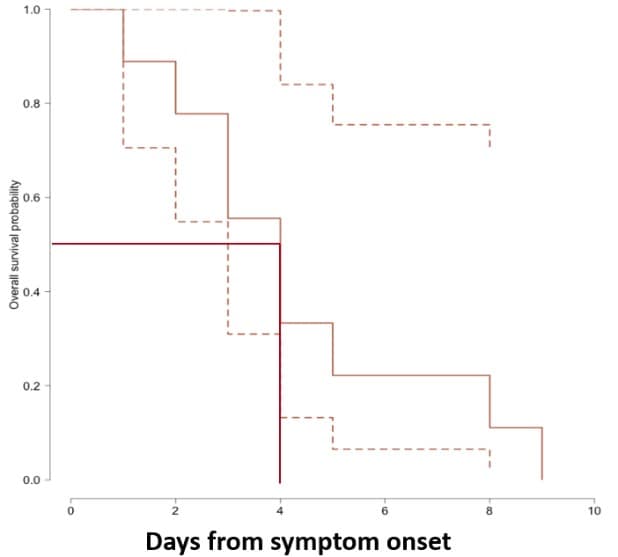
Figure 3. From van Kampen et al., demonstrating declining viral RNA loads (Log10 RNA copies/mL) and likelihood of positive viral culture for SARS-CoV-2 in the upper respiratory samples from a sample of severely ill patients, including some post -solid organ or -bone marrow transplant. Black boxes represent samples that yielded replication-competent virus.
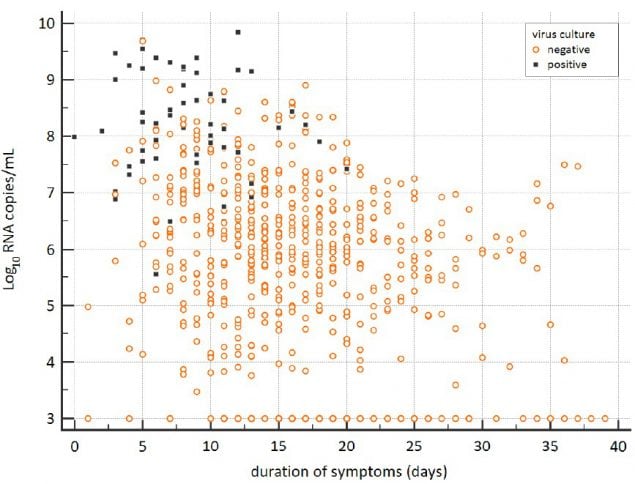
Figure 4. From van Kampen et al., demonstrating decreasing estimated probability of positive viral culture for SARS-CoV-2 from upper respiratory specimens among severely ill patients with COVID-19 with increasing days since symptom onset (upper panel) and decreasing viral load as measured by RT-PCR on the same specimens (lower panel).
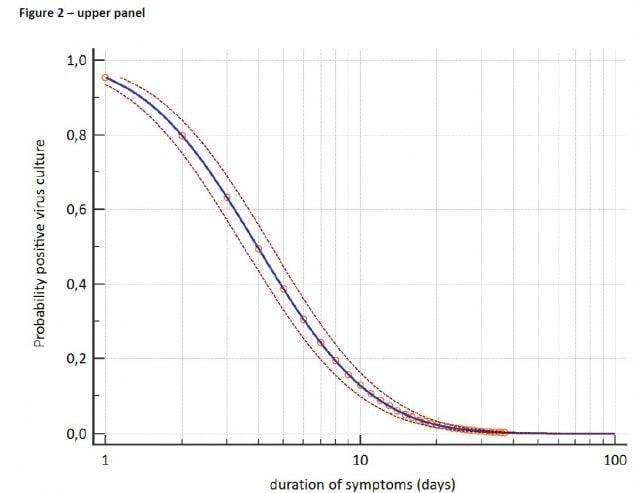
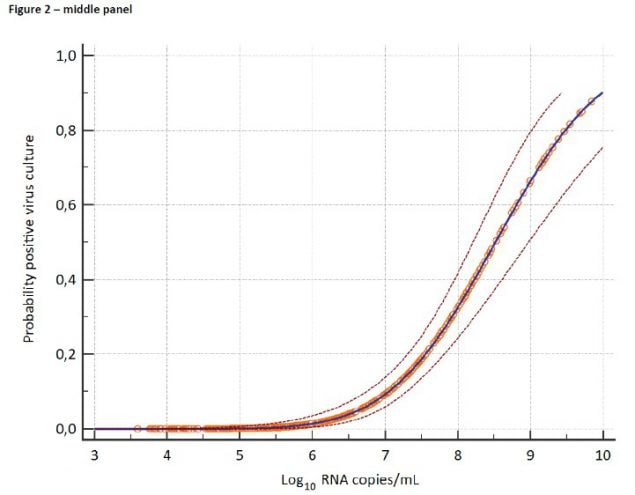
Figure 5. CDC unpublished data showing median Ct values and their 95% confidence intervals among specimens from which replication-competent virus was recovered and not recovered according to the Ct value for the amplification target (N1, N2, or N3) in the CDC RT-PCR assay. RNP = human RNase P, a positive control for the presence of adequate human sample. Red dots indicate specimens with inconclusive RT-PCR amplification according to their corresponding Ct values and culture results.
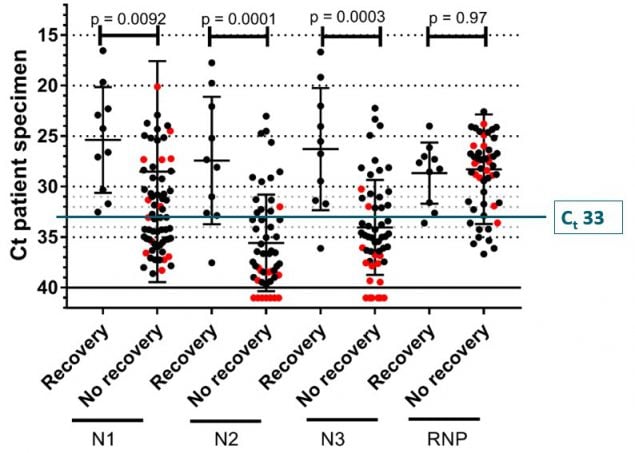
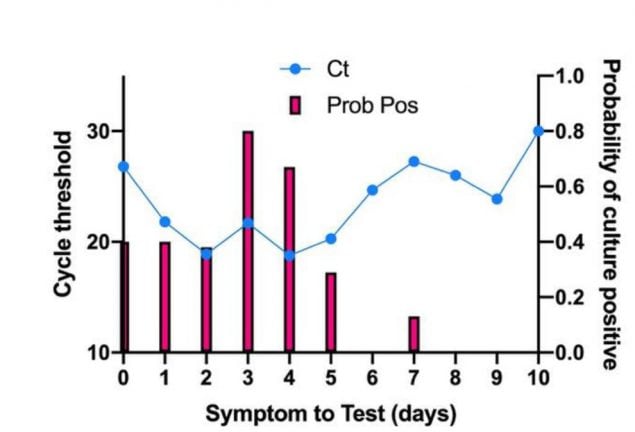
Figure 6. From Bullard et al., comparing symptom onset to test (days) to the probability of successful culture on Vero cells (bar graph) and SARS-CoV-2 E gene RT-PCR cycle threshold (Ct) value (line graph).





















.png)












No hay comentarios:
Publicar un comentario