
NIAID Scientists Reveal How a “Closed” Protein Structure Helps HIV Dodge Antibodies
NIAID Now | January 17, 2019

HIV is a master of sneaking past our natural defenses. Typically, when the body encounters a harmful virus, immune cells recognize viral proteins and stimulate the production of protective proteins called antibodies. These antibodies mount a powerful immune response that clears the virus and protects the body from future infection. HIV, however, has evolved several ways to cloak vulnerable areas of viral proteins that antibodies might otherwise recognize. A report released last month from NIAID scientists in the journal MBio reveals new insights into one such tactic of immune invisibility.
The findings center on an important protein on the surface of HIV, or envelope protein, known as Env. After the discovery of HIV, scientists noticed that Env plays a critical role in establishing HIV infection in the body. A region of the protein connects with a receptor on the surface of certain human T cells, allowing the virus to enter the cell and eventually replicate. Env slips past the immune system in part because of its clever shape: three identical subunits arrange so that Env’s sugar-coated surface faces the environment while protein areas immune cells can recognize, called epitopes, are sheltered within the “closed” structure. The immune system is much less likely to recognize sugars than proteins, and the physical arrangement of Env—resembling the closed petals of a crocus flower at night—keeps the vulnerable epitopes hidden away from lurking antibodies.
In 2014, a team of NIAID scientists in the Laboratory of Immunoregulation, led by Dr. Paolo Lusso, discovered that two specific, small building blocks of the large protein play a very important role in holding together the protein’s closed configuration. The team identified these building blocks, called amino acids, as two tyrosines with unique properties that allowed them to act as a “plug” that prevented Env from opening into a shape that would otherwise be more favorable in its environment.
In the newly reported experiments, Dr. Lusso and his colleagues genetically altered various strains of HIV to express different amino acids—alanine and phenylalanine—where the two influential tyrosines would normally be. The mutant versions of Env indeed lost their tightly closed shapes, now resembling blooming crocuses.
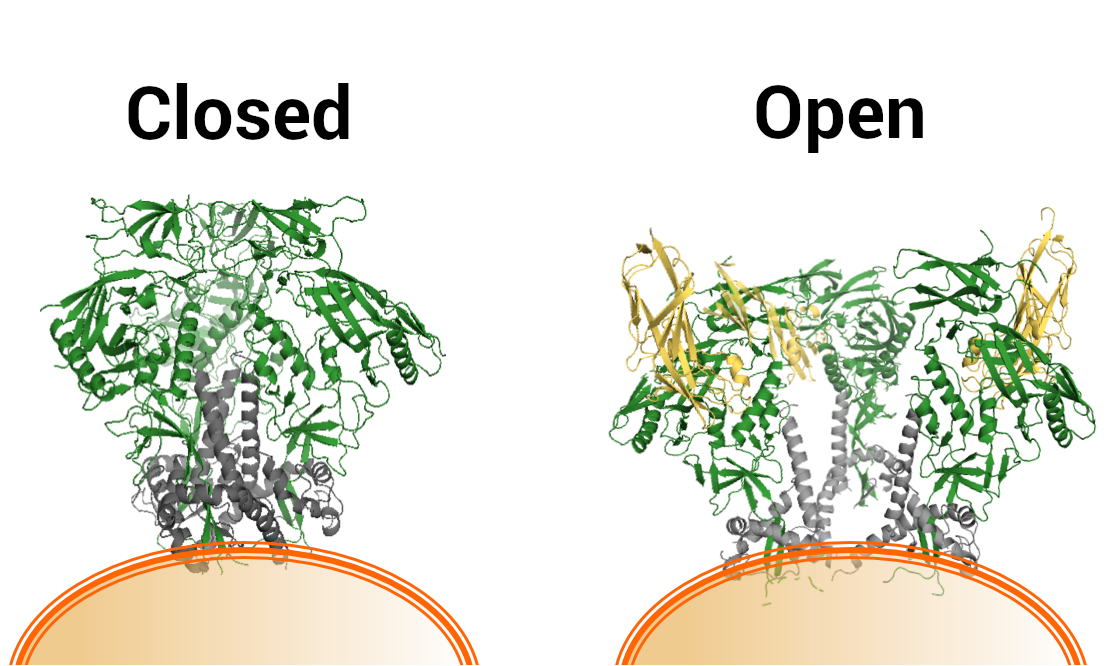
A laboratory illustration depicts the differing structures of the naturally “closed” Env protein on the surface of HIV and a genetically mutated, “open” variant.
Credit: NIAID
The team then systematically exposed both the natural HIV and the genetically mutated viruses to blood samples from people living with HIV. The researchers found that these samples contained anti-HIV antibodies that were able to neutralize the mutant Env but not natural, closed Env. The results help confirm that most antibodies that people living with HIV produce are indeed unable to pierce Env’s clever shield and that two essential tyrosines are responsible for holding it together.
Unfortunately, scientists cannot genetically alter HIV a person has already acquired to become more vulnerable to antibodies that are already present in their body. However, this research may inspire new approaches to target this critical region of Env, which is itself an important target of experimental HIV vaccines. Ultimately, these insights may help vaccine researchers design more effective candidates by offering a clearer understanding of a crafty virus.
Reference:
C. Guzzo et al. Structural constraints at the trimer apex stabilize the HIV-1 envelope in a closed, antibody-protected conformation. MBio DOI: 10.1128/mBio.00955-18 (2018).


































No hay comentarios:
Publicar un comentario