
Reducing Anemia in Developing Regions with Point of Care Analysis
 insights from industryKatja LemburgGlobal Product Manager of HematologyEKF Diagnostics
insights from industryKatja LemburgGlobal Product Manager of HematologyEKF DiagnosticsPublic health programs often use point of care analyzers to assess at risk patients. Please give an overview of health programs you are aware of that use POC hemoglobin analyzers and the work that they do.
The burden of anemia is recognized worldwide, with a global prevalence of about 25%, rising to more than 60% in some developing countries. To address this a multitude of public and NGO funded health programs are working towards achieving the ‘Second Global Nutrition Target 2025’ - a 50% reduction of anemia in women of reproductive age, as outlined in the Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutrition and endorsed by the 65th World Health Assembly (resolution WHA65.6).
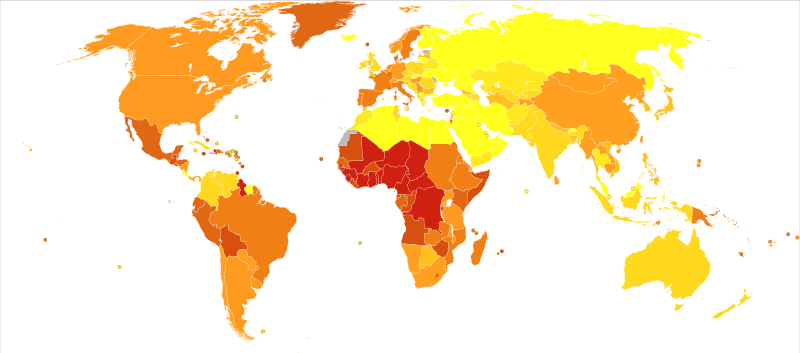

Deaths from Iron-deficiency anaemia in 2012 per million persons. Statistics from WHO, grouped by deciles. CC 4.0.
These programs, mostly referred to as anemia screening programs, are often an integral part of larger nutrition programs. Malnutrition is one of the major causes of anemia; not necessarily because of a lack of nutrition, but because of diets lacking substances like iron, folate and vitamin B12 which are essential for generating hemoglobin.
Anemia screening programs assess the baseline prevalence of anemia in a population. They help with supplementation and fortification and monitor the effectiveness of the nutrition and health program, not only helping the individual but also generating statistics and constantly improving programs by reviewing the outcome. The measurement of hemoglobin is repeatedly performed in this process.
Some health programs are linked to specific disease states with impact on the hemoglobin status, like HIV, malaria and parasite infections, which are most common in developing countries
Besides the geographical spread anemia is often linked to the social economic status of an individual. Anemia is a problem of the poor and underserved and the most vulnerable population groups are women, infants and children.
Why are POC practices such a good fit for these programs? What are the underlying requirements?
POC practices mostly use minimally invasive sampling techniques like finger pricks which can be performed in any setting. POC analyzers are compact in design and are factory calibrated with permanent calibration. This minimizes the need for lab personnel to be present for their operation. Regular health care staff can perform the test and only minimal training is required.
All consumables are single use, can be brought to the test site, and because POC analyzers deliver rapid results, a high number of patients can be tested within a short period.
Whilst fast results are important it is crucial that POC analyzers provide accurate results which are traceable to established laboratory reference methods. Only then can the results be used to support clinical decisions on-site and deliver reliable information for the statistical analysis of screening programs.
Often health programs are conducted in rural and hard to reach regions, what advantages do POC analyzers provide in these areas?
Mobility and robustness are the key advantages of POC analyzers for use in rural and hard to reach regions. For example, POC analyzers usually operate with internal batteries and, in rural areas, where the absence of electricity or frequent outages are a common problem, this ‘self-sufficiency’ is vital.
All materials for the health program need to be transported by truck or air to reach the test sites and a wide range of temperatures and humidity can be experienced. The POC analyzer and its related consumables need to be designed to endure these conditions.
What features have EKF included to ensure that the DiaSpect Tm is robust in potentially harsh environments?
The DiaSpect Tm has been specifically designed with rural and difficult to reach regions in mind. The analyzer and cuvettes can be stored at 0° to 50°C and can withstand temperatures of -30°C to 70°C during transport for a maximum of 24 hours. The DiaSpect Hemoglobin Cuvettes are reagent-less and in contrast to other reagent-based hemoglobin cuvettes, are not affected by humidity.
The DiaSpect Tm analyzer is lightweight (180 g) and compact with no moving parts. It can be used as a hand-held and a robust plastic carrying case is available to ensure safe transportation.
Besides the performance requirements on the POC analyzer the demands for electronic data storage and documentation are increasing, even in outreach projects. EKF has recently launched its POC Connect app to provide a practical and portable data management tool for remote use.
POC Connect is a simple android application that enables users to store, access and transmit hemoglobin results directly from the DiaSpect Tm device to a smartphone via integrated Bluetooth® technology. It allows the user to add patient and operator details, comments and note the materials in use.
This data is held in a history file on a smartphone and can be transferred to a central database once an internet connection is available or by using a sufficiently strong mobile network. The lack of permanently available internet infrastructure and traditional land-line installations can be overcome this way.
How does FDA clearance and CLIA Waived status resonate and improve acceptance of the device on other public health programs globally?
Besides opening new opportunities in the USA there is an increasing recognition of FDA clearance and CLIA Waived status in many other countries. US based institutions like CAP and JCI offer their accreditations globally making their standards commonly available.
Some countries, for example those in the Middle East are progressing towards only importing medical products and IVDs which have FDA clearance. In other countries it helps to speed up the processes of local registrations.
CLIA Waived status is seen as confirmation of the ease of use of a system which is an essential requirement especially during field use in rural areas or in other non-clinical environments.
How can EKF Diagnostics’ new DiaSpect Tm hemoglobin analyzer help the USA WIC program specifically? What make this product ideal for this type of program?
The USA WIC program is a Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and provides federal grants to states for supplemental food, health care referrals, and nutrition education for low-income pregnant, breastfeeding, and non-breastfeeding postpartum women, as well as to infants and children up to age five who are found to be at nutritional risk.
Participants in the WIC program are tested for hemoglobin on a regular basis as part of the health service and nutrition advisory activities. Typically, the WIC offices are non-clinical settings, often situated in neighborhood centers.
The DiaSpect Tm system is ideal for such settings due to its small footprint, ease of use and the long shelf-life of consumables. The results are available within seconds and are traceable to a laboratory reference method.
Why is it so important for countries to run effective preventative healthcare programs such as WIC?
Anemia reduces an individual’s wellbeing, physical productivity and work performance. It has a negative impact on development and the learning capabilities of children. Maternal anemia is associated with increased mortality and morbidity in the mother and baby, including the risk of miscarriages, stillbirths, prematurity and low birth weight.
A series of reports published by the USDA based on linked 1988 WIC and Medicaid data on over 100,000 births found that for every dollar spent on prenatal WIC participation for low-income women on Medicaid in five states resulted in savings in health care costs of between $1.77 and $3.13 within the first 60 days after birth and a significant reduction of risks associated with anemia.
What is the future for EKF’s POC analyzers?
Whilst the United States currently accounts for almost 50% of the total POC market, followed by regions such as Europe and Japan, we expect to see an increase of POC analyzers being used in emerging countries that have improving healthcare but a limited infrastructure for stationary laboratory systems and sample rotation.
Furthermore, the use of POC analyzers is very common in the monitoring and treatment of lifestyle related disorders, such as diabetes. Future initiatives will aim at prevention of disease states rather than treating avoidable diseases later, and POC analyzers will play an important role in this approach giving the possibility to test patients-at-risk in preventive health checks which could be established for example in community settings, gyms, nursing homes or pharmacies.
Ideally, medical information from such health program should be interfacing with clinical patient records and also with health insurances that could incentivize participation and offer reimbursement. This would require embedding the POC analyzer in data communication networks ensuring an efficient and at the same time secure transfer of medical data. Mobile connectivity solutions could become a key element in this setting.
EKF Diagnostics’ current POC analyzer offering is focused on diabetes, anemia and sports and an expanding use in nutrition and exercise programs can be expected. Mobile connectivity solutions will be developed further to support the use in a decentralized health infrastructure, making our products fit for a healthier life.
Where can readers find more information?
- www.ekfdiagnostics.com
- EKF DiaSpect Tm Advanced Training: https://www.youtube.com/watch?v=GmDalpDclAQ
- www.who.int/topics/anaemia/en
- www.fns.usda.gov/wic/about-wic-how-wic-helps
- RNCOS Market Research Report “Global Point-of-Care Diagnostics Market Outlook 2018”
About Katja Lemburg
Global Product Manager Hematology of EKF Diagnostics - Katja Lemburg graduated in Biomedical Engineering at the University of Applied Sciences Luebeck, Germany in 1997 with a focus on development of biosensors. After several years of working in sales and service of clinical research products, she joined EKF Diagnostics as Product Manager in 2008.



































No hay comentarios:
Publicar un comentario