Flipping a Genetic Switch on Obesity?
 When weight loss is the goal, the equation seems simple enough: consume fewer calories and burn more of them exercising. But for some people, losing and keeping off the weight is much more difficult for reasons that can include a genetic component. While there are rare genetic causes of extreme obesity, the strongest common genetic contributor discovered so far is a variant found in an intron of the FTO gene. Variations in this untranslated region of the gene have been tied to differences in body mass and a risk of obesity [1]. For the one in six people of European descent born with two copies of the risk variant, the consequence is carrying around an average of an extra 7 pounds [2].
When weight loss is the goal, the equation seems simple enough: consume fewer calories and burn more of them exercising. But for some people, losing and keeping off the weight is much more difficult for reasons that can include a genetic component. While there are rare genetic causes of extreme obesity, the strongest common genetic contributor discovered so far is a variant found in an intron of the FTO gene. Variations in this untranslated region of the gene have been tied to differences in body mass and a risk of obesity [1]. For the one in six people of European descent born with two copies of the risk variant, the consequence is carrying around an average of an extra 7 pounds [2].Now, NIH-funded researchers reporting in The New England Journal of Medicine [3] have figured out how this gene influences body weight. The answer is not, as many had suspected, in regions of the brain that control appetite, but in the progenitor cells that produce white and beige fat. The researchers found that the risk variant is part of a larger genetic circuit that determines whether our bodies burn or store fat. This discovery may yield new approaches to intervene in obesity with treatments designed to change the way fat cells handle calories.
The team—led by Melina Claussnitzer of Beth Israel Deaconess Medical Center, Boston, and Manolis Kellis of the Massachusetts Institute of Technology (MIT), Cambridge—started with a basic question: where in the body does this variant act to influence weight? For the answer, the team turned to the NIH-funded Roadmap Epigenomics Project. There, they found comprehensive data on 127 human cell types and the occurrence of common chemical modifications that act like volume knobs to turn gene activity “up” or “down” based on changes in the way DNA is packaged. While the FTOgene is active in the human brain, the team couldn’t connect any differences there with obesity.
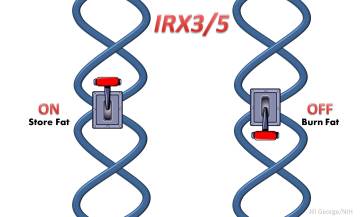
They began to wonder whether this obesity-risk variant affected FTO at all (and prior studies had suggested this [4]). Maybe it operated at a distance to change the expression of other protein-coding genes? Sure enough, further study in fat collected from patients showed that the obesity risk variant works in those progenitor cells to control the activity of two other genes, IRX3 andIRX5, both found quite a distance away.
The fat in people with the obesity risk variant and greater expression of IRX3 and IRX5 genes contains fewer beige cells than normal. Beige cells, which were discovered just three years ago [5], are produced sometimes by fat cell progenitors to burn rather than stockpile energy. This new evidence suggests that beige fat may play an unexpectedly important role in protecting against obesity.
Using a method they developed last year [6], the researchers traced the effects of the obesity risk variant to a single nucleotide change—a small typo in the DNA sequence that changes a “T” to a “C.” They then used the nifty CRISPR-Cas genome editing system (see Copy-Editing the Genome) to switch between this obesity risk variant and the protective variant in human cells. As the researchers did this, they saw fat cells turn energy-burning heat production off and back on again. In other words, the obesity signature in the cells could be turned on and off at the flip of this genetic switch!
They also showed in mice that the shift toward energy-burning beige cells led to weight loss. Animals engineered in a way that blocked Irx3 expression in adipose tissue became significantly thinner with no change in their eating or exercise habits. This new collection of evidence suggests that treatments designed to program fat cells to burn more energy (such as antagonists against the IRX3 or IRX5 proteins) might have similar benefits in people, and the researchers are working with collaborators in academia and industry to pursue this line of investigation.
This is a great example of how discoveries about genetic factors in common disease, uncovered by applying the genome-wide association study (GWAS) approach to large numbers of affected and unaffected individuals, are revealing critical and previously unknown pathways in human biology and medicine. This case also points out how our terminology may need attention, however; for the last several years, this genetic variant for obesity has been called “the FTO variant,” perhaps it should now be called “the IRX3/5 variant.”
Genes, of course, are only part of the story. It’s still important to eat healthy, limit your portions, and maintain a regular exercise program. Leading an active lifestyle both keeps weight down and improves the overall sense of well being.
References:
[1] FTO genotype is associated with phenotypic variability of body mass index.Yang J, Loos RJ, Powell JE, TM, Frayling TM, Hirschhorn JN, Goddard ME, Visscher PM, et al. Nature. 2012 Oct 11;490(7419):267-72.
[2] A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Frayling TM, Timpson NJ, Weedon MN, Morris AD, Smith GD, Hattersley AT, McCarthy MI, et al. Science. 2007 May 11;316(5826):889-94.
[3] FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, Glunk V, Sousa IS, Beaudry JL, Puviindran V, Abdennur NA, Liu J, Svensson PA, Hsu YH, Drucker DJ, Mellgren G, Hui CC, Hauner H, Kellis M. N Engl J Med. 2015 Aug 19. [Epub ahead of print]
[4] Obesity-associated variants within FTO form long-range functional connections with IRX3. Smemo S, Tena JJ, Kim KH, Hui CC, Gomez-Skarmeta JL, Nobrega MA, et al. Nature 2014 Mar 20; 507(7492):371-375.
[5] Beige adipocytes are a distinct type of themogenic fat cell in mouse and human. Wu J, Boström P, Sparks LM, Schrauwen P, Spiegelman BM. Cell 2012 Jul 20:150(2):366-376.
[6] Leveraging cross-species transcription factor binding site patterns: from diabetes risk loci to disease mechanisms. Claussnitzer M, Dankel SN, Klocke Mellgren G, Hauner H, Laumen H, et al. Cell. 2014 Jan 16;156(1-2):343-58.
Links:
Manolis Kellis (Massachusetts Institute of Technology, Cambridge)
What are overweight and obesity? (National Heart, Lung, and Blood Institute/NIH)
NIH Support: National Human Genome Research Institute; National Institute of General Medical Sciences





















.png)












No hay comentarios:
Publicar un comentario