A new DRUG TRIALS SNAPSHOT is now available.
Drug Trials Snapshots: BEOVU

BEOVU is a treatment for neovascular (wet) Age-Related Macular Degeneration (AMD).
AMD is a disease of the retina - a layer of the eye that contains cells which are sensitive to light. In wet AMD, the macula (small central area of the retina) is damaged due to the growth of new blood vessels. At first, this damage causes blurred vision and then progressive vision loss.
BEOVU is an injection. It is injected by a healthcare professional directly inside the eyeball every month for three months and then every 8-12 weeks.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
BEOVU (brolucizumab-dbll)
bee oh' voo
Novartis
Approval date: October 7, 2019
bee oh' voo
Novartis
Approval date: October 7, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BEOVU is a treatment for neovascular (wet) Age-Related Macular Degeneration (AMD).
AMD is a disease of the retina - a layer of the eye that contains cells which are sensitive to light. In wet AMD, the macula (small central area of the retina) is damaged due to the growth of new blood vessels. At first, this damage causes blurred vision and then progressive vision loss.
How is this drug used?
BEOVU is an injection. It is injected by a healthcare professional directly inside the eyeball every month for three months and then every 8-12 weeks.
What are the benefits of this drug?
After one year of treatment, patients treated with BEOVU showed similar visual improvement as patients treated with an already approved drug for wet AMD called aflibercept.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
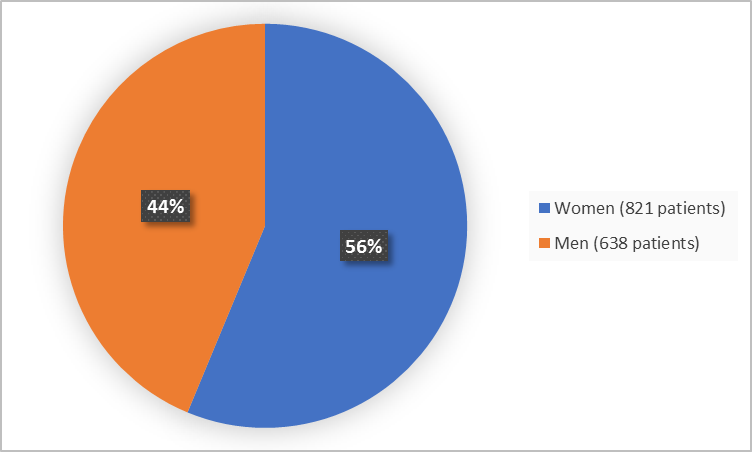
- Sex: BEOVU worked similarly in men and women.
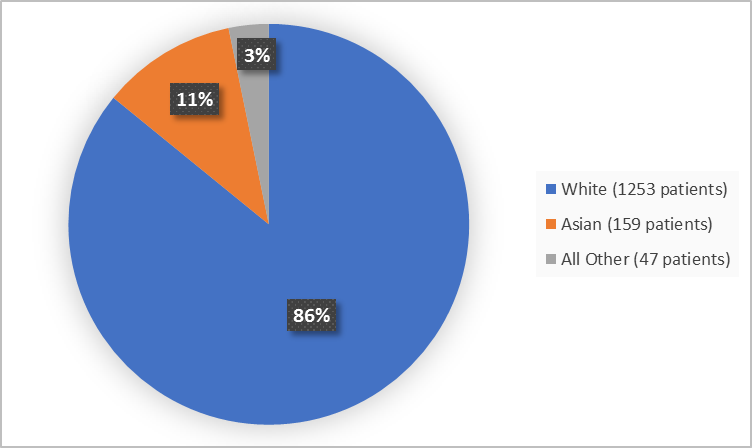
- Race: BEOVU worked similarly in White and Asian patients. Differences in how well BEOVU worked among other races could not be determined because of the limited number of patients.
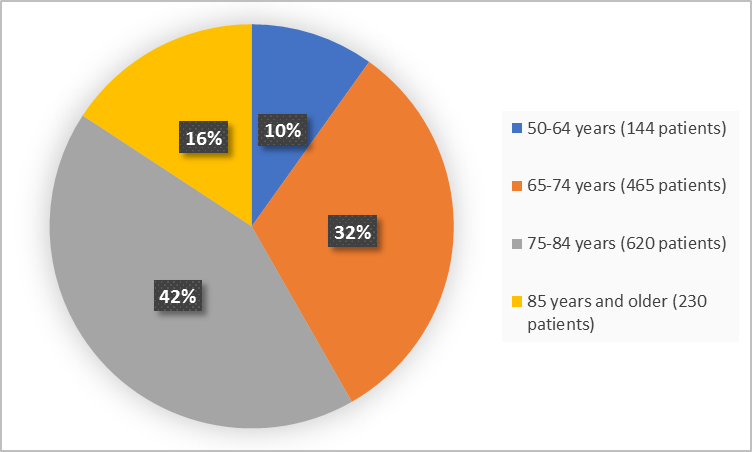
- Age: BEOVU worked similarly in all age groups including the elderly.
What are the possible side effects?
BEOVU may cause serious side effects including:
- eyeball infection
- eyeball inflammation
- retinal detachment
- increase of the eyeball pressure
- forming blood clots in blood vessels
The most common side effects are decreased visual acuity, cataracts, conjunctival bleeding, eye inflammation and eye floaters.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in White and Asian patients. Differences in the occurrence of side effects among other races could not be determined because of the limited number of patients.
- Age: The occurrence of side effects was similar in all age groups including the elderly.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved BEOVU based on evidence from two clinical trials (Trial 1/ NCT02307682 and Trial 2/NCT02434328) of 1459 patients, 50-97 years old, with wet AMD. The trials were conducted at 336 of sites in US, Canada, Central and South America, European countries, Israel, Turkey, Australia, New Zealand, Japan, South Korea, Singapore, Taiwan, and Vietnam.
Figure 1 summarizes how many men and women were enrolled in the clinical trials used to evaluate efficacy.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trials used to evaluate efficacy.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 1253 | 86 |
| Asian | 159 | 11 |
| Black or African American | 3 | Less than 1 |
| American Indian or Alaska Native | 2 | Less than 1 |
| Other | 35 | 2 |
| Multiple | 7 | 1 |
FDA Review
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
There were two trials that evaluated the benefits and side effects of BEOVU in patients with wet AMD. Approximately half of the patients were treated with BEOVU and the other half with aflibercept (an approved drug for wet AMD treatment) for two years. Both drugs were injected into the eyeball by physicians and neither the patients nor the physicians knew which treatment was being given until after the trial was completed.
The benefit was assessed by measuring the change in vision acuity in patients treated with BEOVU versus patients treated with aflibercept for the duration of the trial. Vision acuity was measured using a standard letter chart.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.





















.png)












No hay comentarios:
Publicar un comentario