A new DRUG TRIALS SNAPSHOT is now available

Drug Trials Snapshots: AKLIEF
AKLIEF is a drug used on the skin to treat acne vulgaris in patients 9 years and older.
Acne vulgaris is a skin disease characterized by blackheads, whiteheads, pimples, and sometimes oily skin and scarring.
AKLIEF is a cream applied to the affected skin of face and/or trunk once a day, in the evening.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
AKLIEF (trifarotene)
ack-LEEF
Galderma Laboratories
Approval date: October 4, 2019
ack-LEEF
Galderma Laboratories
Approval date: October 4, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
AKLIEF is a drug used on the skin to treat acne vulgaris in patients 9 years and older.
Acne vulgaris is a skin disease characterized by blackheads, whiteheads, pimples, and sometimes oily skin and scarring.
Acne vulgaris is a skin disease characterized by blackheads, whiteheads, pimples, and sometimes oily skin and scarring.
How is this drug used?
AKLIEF is a cream applied to the affected skin of face and/or trunk once a day, in the evening.
What are the benefits of this drug?
More patients achieved a reduction in the number of acnes and clear or almost clear skin after 12 weeks of treatment with AKLIEF cream in comparison to those who were treated with placebo cream.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: AKLIEF worked similarly in males and females.
- Race: AKLIEF worked similarly among tested races.
- Age: AKLIEF worked similarly between patients younger and older than 18 years of age.
What are the possible side effects?
The most common side effects of AKLIEF are application site reactions (irritation and itching), and sunburn. Sometimes, these side effects could be severe.
Patients are advised to minimize sunlight and ultraviolent light exposure.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar among males and females.
- Race: The occurrence of side effects was similar among tested races.
- Age: The occurrence of side effects was similar between patients younger and older than 18 years of age.
WHO WAS IN THE CLINICAL TRIAL?
Who participated in the clinical trials?
The FDA approved AKLIEF based primarily on evidence from two clinical trials (Trial 1/NCT02566369 and Trial 2/NCT02556788) of 2420 patients with moderate acne vulgaris on the face and/or trunk. The trials were conducted at 198 sites in the United States, Canada, Puerto Rico and Europe.
Demographics of these two trials are presented in Figures 1-3 below and in in Table 6 under MORE INFO.
One additional trial of 453 patients with moderate acne vulgaris on the face and/or trunk assessed the side effects when AKLIEF was used for one year. Demographics of that trial is presented separately in Table 7 under MORE INFO.
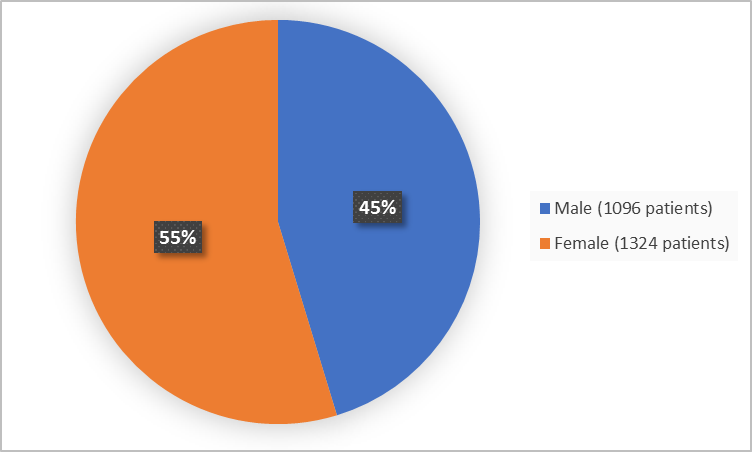
Figure 1 summarizes how many males and females were in the clinical trials 1 and 2.
Baseline Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trials 1 and 2.
Figure 2. Baseline Demographics by Race
*Other includes American Indian or Alaska Native, Missing
FDA Review
Table 1. Demographics of Trials 1 and 2 by Race
Race | Number of Patients | Percentage of Patients |
|---|---|---|
White | 2111 | 87 |
Black or African American | 165 | 7 |
Asian | 63 | 3 |
American Indian or Alaska Native | 19 | 1 |
Native Hawaiian or Other Pacific Islander | 3 | Less than 1 |
Multiple | 22 | 1 |
Other | 37 | 1 |
FDA Review
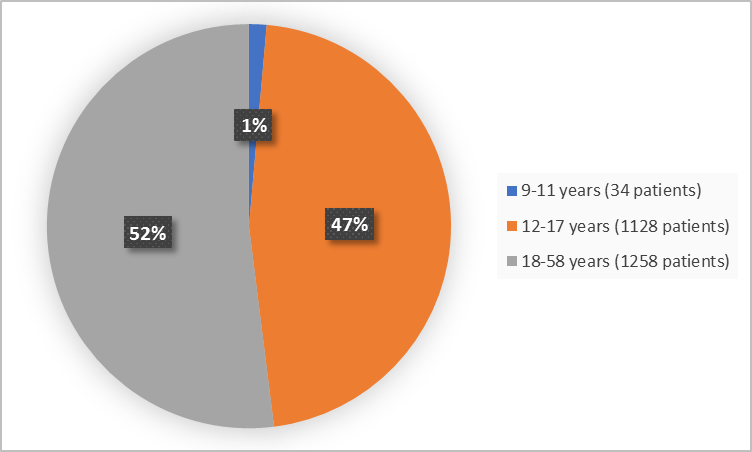
Figure 3 summarizes the percentage of patients by age in the clinical trials 1 and 2.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
There were three trials that were used to approve AKLIEF for treatment of acne in patients 9 years and older.
Trials 1 and 2 enrolled patients 9 years of age and older with moderate facial and truncal acne vulgaris. Approximately half of all patients applied AKLIEF cream and the other half applied placebo cream once daily (at night) for 12 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. The benefit of AKLIEF in comparison to placebo was assessed after 12 weeks of treatment using the Investigator’s Global Assessment (IGA) score that measures the severity of disease (on a scale from 0 to 4) and a decrease in the number of pimples.
Trial 3 enrolled patients 9 years and older with moderate facial and/or truncal acne vulgaris. Patients received AKLIEF cream once daily for one year. Patients in Trial 3 were primarily evaluated for side effects.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.






















.png)












No hay comentarios:
Publicar un comentario