A new DRUG TRIALS SNAPSHOT is now available.

NUBEQA is a drug for the treatment of prostate cancer that has not spread to other parts of the body (non-metastatic) and no longer responds to a medical or surgical treatment that lowers testosterone (castration-resistant).
NUBEQA is a tablet. Two tablets are taken once daily with food.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: NUBEQA
NUBEQA (darolutamide)
NOO-bƏ-kƏ
Bayer
Approval date: July 30, 2019
NOO-bƏ-kƏ
Bayer
Approval date: July 30, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
NUBEQA is a drug for the treatment of prostate cancer that has not spread to other parts of the body (non-metastatic) and no longer responds to a medical or surgical treatment that lowers testosterone (castration-resistant).
How is this drug used?
NUBEQA is a tablet. Two tablets are taken once daily with food.
What are the benefits of this drug?
In patients with medical or previous surgical treatment to lower testosterone, NUBEQA increased the patients’ survival during which time the cancer did not spread [metastasis-free survival (MFS)]. The MFS for patients taking NUBEQA was about 40 months compared to about 18 months for patients taking a placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
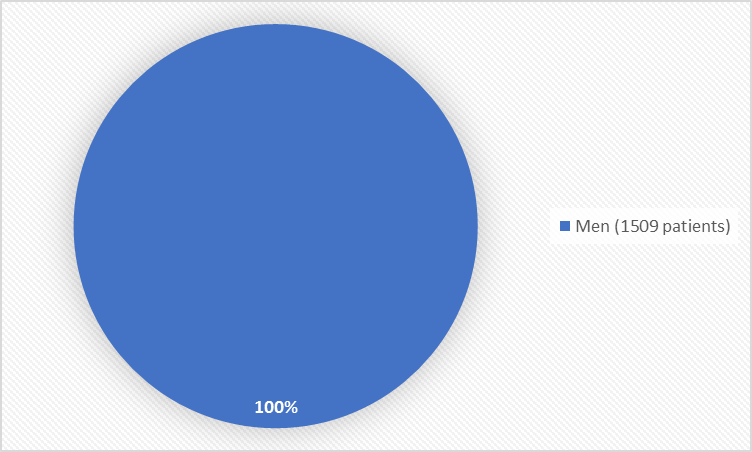
- Sex: All the patients were men since NUBEQA is for the treatment of prostate cancer.
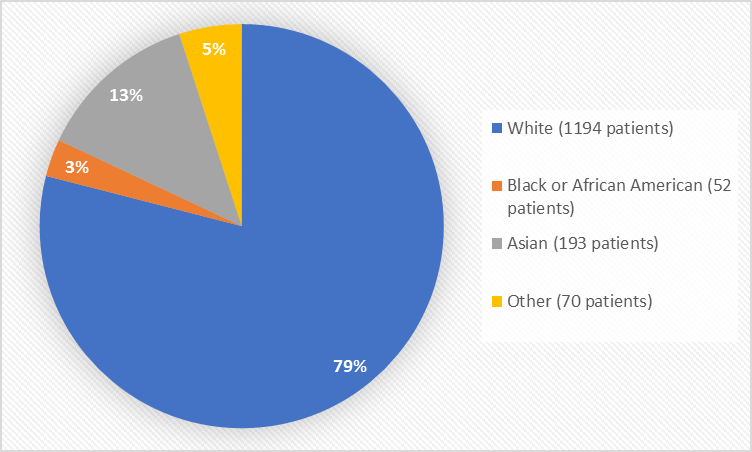
- Race: The majority of patients were White. NUBEQA worked similarly in White and Asian patients. The number of patients in other races was limited; therefore, differences in how well NUBEQA worked among races could not be determined.
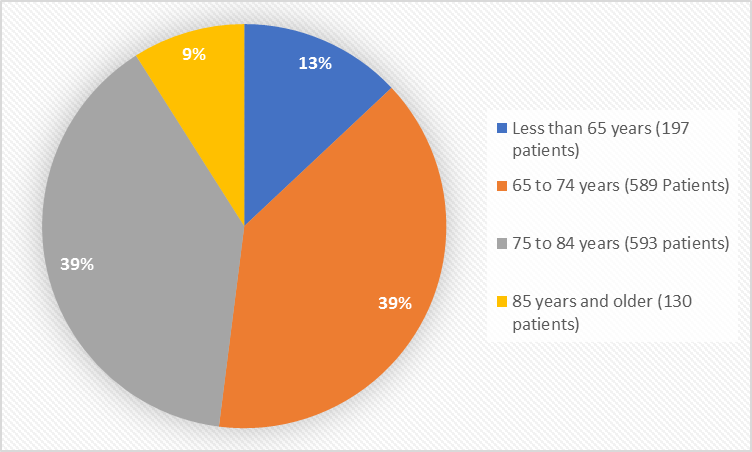
- Age: NUBEQA worked similarly in all age groups tested.
What are the possible side effects?
NUBEQA can cause harm to a fetus and loss of pregnancy in female sexual partners.
The most common side effects are fatigue, pain in extremity, and rash.
Were there any differences in side effects among sex, race and age?
- Sex: All the patients were men since NUBEQA is for the treatment of prostate cancer.
- Race: The occurrence of side effects was similar among White and Asian patients. The number of patients in other races was limited; therefore, the occurrence of side effects among other races could not be determined.
- Age: The occurrence of side effects was similar in all age groups tested.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved NUBEQA based on evidence from one clinical trial (NCT02200614) of 1509 patients with non-metastatic, castrate-resistant prostate cancer.
The trial was conducted in Asia Pacific, Australia, Canada, Europe, Latin America, South Africa, and the United States.
Figure 1 summarizes how many men were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Clinical Trial by Race
[table or other code]
Race | Number of Patients | Percent |
White | 1194 | 79 |
Black or African American | 52 | 3 |
Asian | 193 | 13 |
Other | 15 | 1 |
Missing | 55 | 4 |
FDA Review
Figure 3 summarizes the percentage of patients by age groups in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
The benefit and side effects of NUBEQA were evaluated in one clinical trial. The trial enrolled men aged 48 to 95 years with prostate cancer that had not spread outside the prostate and that had not responded to castration. Patients were assigned to receive NUBEQA or placebo by mouth twice daily. Neither the patient nor the healthcare provider knew which treatment was given until after the trial was completed. Treatment continued until disease progression or unacceptable side effects. All patients received either hormone therapy or had previous surgery to lower the amount of testosterone in their body.
The benefit of NUBEQA was assessed by measuring the length of time after the start of treatment that the cancer did not spread to other parts of the body and that the patient was still alive (metastasis-free survival [MFS]).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.





















.png)












No hay comentarios:
Publicar un comentario