A new DRUG TRIALS SNAPSHOT is now available.

ACCRUFER is a drug used for the treatment of adults with low iron stores in the body.
ACCRUFER is a capsule taken two times a day on an empty stomach.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: ACCRUFER
ACCRUFER (ferric maltol)
ak-roo-fer
Shield Therapeutics
Approval date: July 25, 2019
ak-roo-fer
Shield Therapeutics
Approval date: July 25, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ACCRUFER is a drug used for the treatment of adults with low iron stores in the body.
How is this drug used?
ACCRUFER is a capsule taken two times a day on an empty stomach.
What are the benefits of this drug?
ACCRUFER was better than placebo in restoring iron stores in the body.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ACCRUFER was similarly effective in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited. Therefore, differences among races in how well the drug worked could not be determined.
- Age: ACCRUFER was similarly effective in patients below and above 60 years of age.
What are the possible side effects?
ACCRUFER can cause serious side effects including increased risk of inflammatory bowel disease flare and iron overload in the body.
The most common side effects of ACCRUFER are gas, diarrhea, constipation, stool color change, nausea, vomiting, and abdominal discomfort, bloating and pain.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited. Therefore, differences in the occurrence of side effects could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 60 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ACCRUFER based on evidence from three clinical trials (Trial 1/ NCT01252221, Trial 2/NCT01340872 and Trial 3/NCT02968368). All 295 patients had low iron stores in the body and consequent iron deficiency anemia. In the first two trials low iron was caused by patients’ inflammatory bowel disease (IBD) and in the last trial, by long standing (chronic) kidney disease or CKD.
Trials were conducted at 79 sites in Europe and the United States.
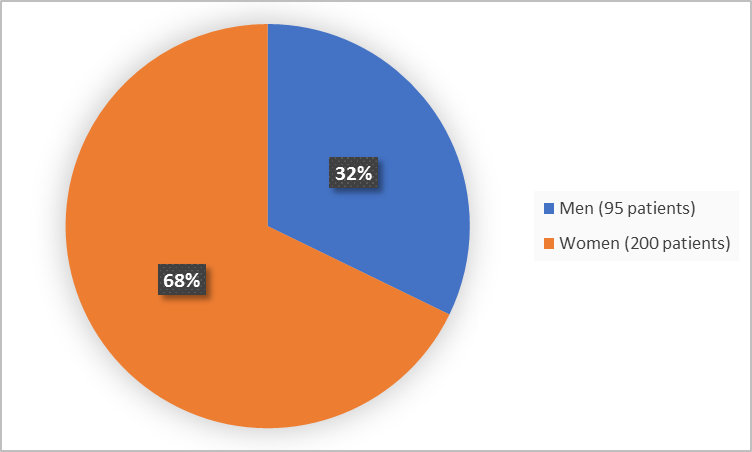
Figure 1 summarizes how many men and women were in the combined clinical trials.
Figure 1. Demographics by Sex (safety population)
FDA Review
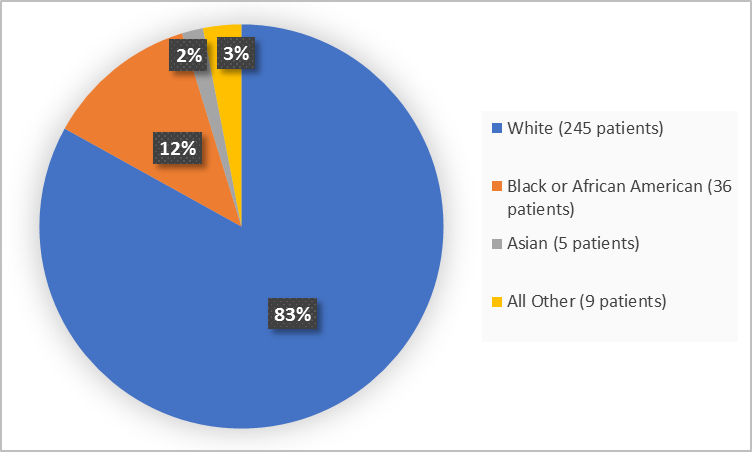
Figure 2 summarizes the percentage of patients by race in the combined clinical trials.
Figure 2. Demographics by Race (safety population)
FDA Review
Table 1. Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 245 | 83 |
| Black or African American | 36 | 12 |
| Asian | 5 | 2 |
| All Other | 9 | 3 |
FDA Review
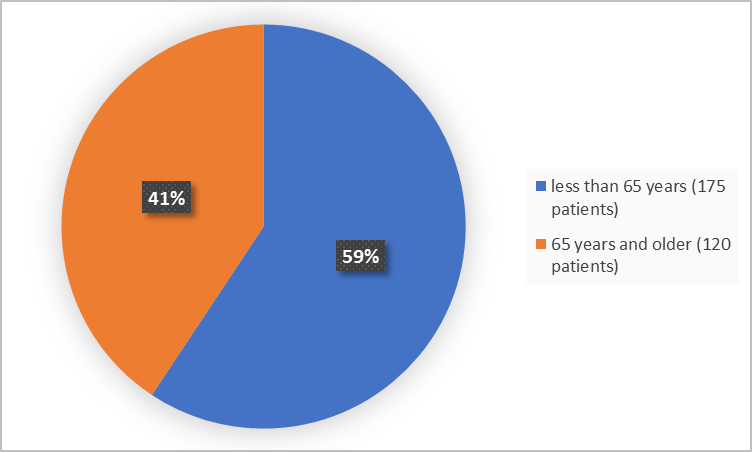
Figure 3 summarizes the percentage of patients by age group in the combined clinical trial.
Figure 3. Demographics by Age (safety population)
Clinical Trial Data
How were the trials designed?
ACCRUFER was evaluated in three clinical trials of 295 patients with low iron stores in the body and iron deficiency anemia as confirmed by blood tests (measuring hemoglobin and ferritin).
In Trials 1 and 2 all patients also had IBS which often causes low iron stores in the body. Patients were randomly assigned to receive either ACCRUFER twice daily or a matched placebo control for 12 weeks. Neither the patients nor the investigators knew which treatment was given until the end of 12 weeks. After 12 weeks, patients from both groups could continue taking ACCRUFER for additional 52 weeks.
In Trial 3 all patients also had CKD which often causes low iron stores in the body. Patients were randomly assigned to receive either ACCRUFER twice daily or a matched placebo control for 16 weeks. Neither the patients nor the investigators knew which treatment was given until the end of 16 weeks.
The benefit of ACCRUFER was assessed by measuring improvement in hemoglobin and iron stores at the end of the treatment and comparing it to placebo.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.





















.png)












No hay comentarios:
Publicar un comentario