A new DRUG TRIALS SNAPSHOT is now available.

KOSELUGO is used to to treat children 2 years of age and older with a type of tumor called plexiform neurofibroma or PN that occurs in a rare disease called neurofibromatosis type 1 (NF1). KOSELUGO is used in patients whose tumors cause symptoms and could not be surgically removed.
KOSELUGO is a capsule taken two times a day.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: KOSELUGO
KOSELUGO (selumetinib)
(ko-SEL-yuh-go)
AstraZeneka
Approval date: April 10, 2020
(ko-SEL-yuh-go)
AstraZeneka
Approval date: April 10, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
KOSELUGO is used to to treat children 2 years of age and older with a type of tumor called plexiform neurofibroma or PN that occurs in a rare disease called neurofibromatosis type 1 (NF1). KOSELUGO is used in patients whose tumors cause symptoms and could not be surgically removed.
How is this drug used?
KOSELUGO is a capsule taken two times a day.
What are the benefits of this drug?
In the trial, 33 of 50 patients (66%) who received KOSELUGO experienced partial tumor shrinkage. Of these patients, 82% had a tumor shrinkage lasting 12 months or longer.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: KOSELUGO worked similarly in males and females.
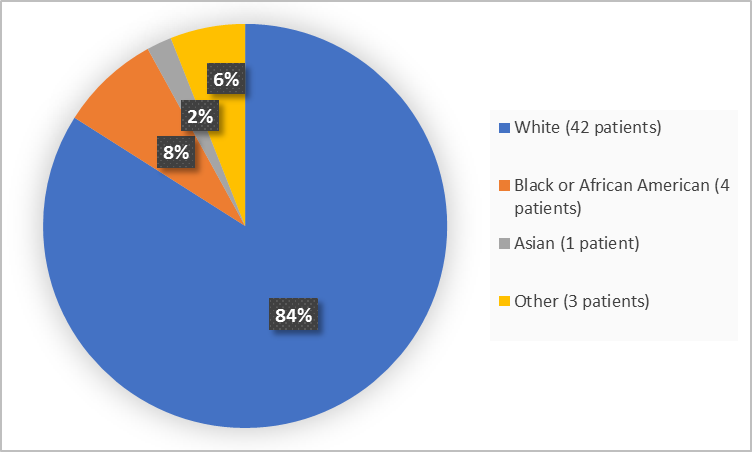
- Race: The majority of patients were White. Therefore, differences among races could not be determined.
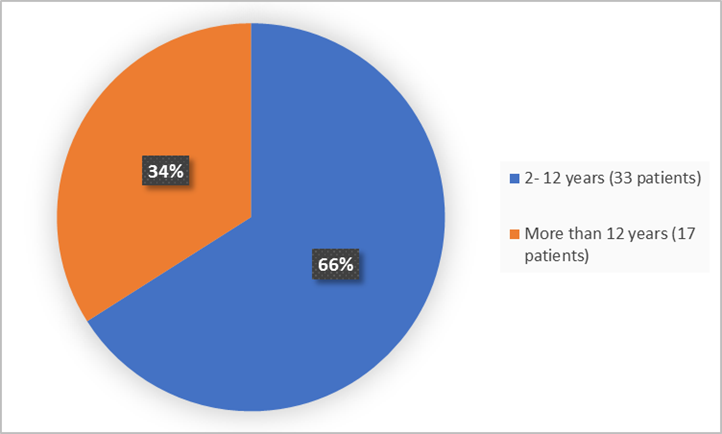
- Age: KOSELUGO worked similarly in children younger and older than12 years of age.
What are the possible side effects?
KOSELUGO may cause serious side effects including:
- weaking of the heart,
- eye problems that can result in loss of sight,
- gastrointestinal problems (diarrhea, bleeding),
- severe skin rashes,
- increased blood levels of enzyme creatinine phosphokinase, and
- increased risk of bleeding when taken with vitam E.
The most common side effects of KOSELUGO are vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, fatigue, muscule and bone pain, fever, acne, mouth sores, headache, nail infection, and itching.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of overall side effects was similar in males and females.
- Race: The majority of patients were White. Therefore, differences in side effects among races could not be determined.
- Age: The occurrence of overall side effects was similar in children younger and older that 12 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved KOSELUGO based on a clinical trial (NCT01362803) of 50 children 2-18 years of age with NF1.
The trial was conducted at 4 sites in the United States.
Figure 1 summarizes how many boys and girls were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 summarizes patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
The figure below summarizes the age groups of patients in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
Figure 4 summarizes patients by ethnicity in the trial.
Figure 4. Baseline Demographics by Ethnicity
FDA Review
How were the trials designed?
The trial enrolled patients with NF1 whose PN(s) were causing significant symptoms and could not be surgically removed. The enrolled patients received KOSELUGO capsule twice daily until disease progression or unacceptable toxicity.
The benefit of KOSELUGO was assessed by the percentage of patients with complete or partial shrinkage of the tumors (overall response rate or ORR).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.























.png)












No hay comentarios:
Publicar un comentario