A new DRUG TRIALS SNAPSHOT is now available.

Drug Trials Snapshots: VYEPTI
VYEPTI is a drug used for the preventive treatment of migraine in adults.
A migraine is a type of headache that can be associated with nausea, vomiting, and/or sensitivity to light or sound.
VYEPTI is an injection given directly into the vein (intravenous infusion) by a healthcare provider every three months. It takes about 30 minutes to receive the VYEPTI infusion.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
VYEPTI (eptinezumab-jjmr)
vye ep' tee
Lundbeck
Approval date: February 21, 2020
vye ep' tee
Lundbeck
Approval date: February 21, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VYEPTI is a drug used for the preventive treatment of migraine in adults.
A migraine is a type of headache that can be associated with nausea, vomiting, and/or sensitivity to light or sound.
How is this drug used?
VYEPTI is an injection given directly into the vein (intravenous infusion) by a healthcare provider every three months. It takes about 30 minutes to receive the VYEPTI infusion.
What are the benefits of this drug?
Over the three-month treatment period, patients treated with VYEPTI experienced fewer days of migraine headaches in comparison to patients who received the placebo treatment.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: VYEPTI worked similarly in men and women.
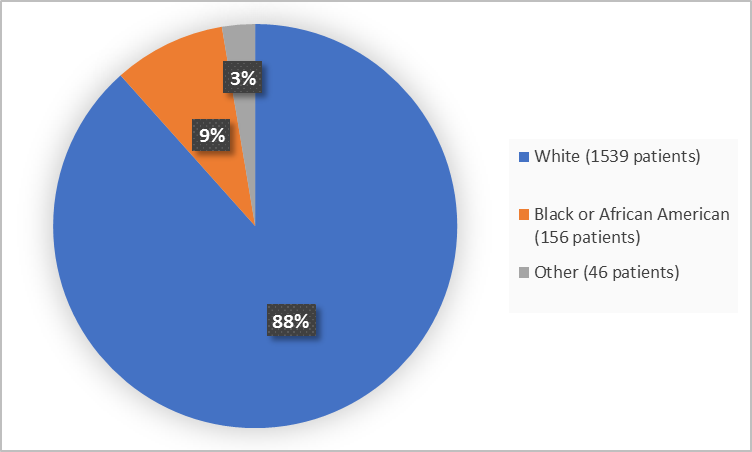
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in how VYEPTI worked among races could not be determined.
- Age: VYEPTI worked similarly in all tested age groups. The number of patients older than 65 years was insufficient to determine if VYEPTI worked differently in this age group.
What are the possible side effects?
VYEPTI may cause serious allergic reactions including swelling of face, tongue or throat, hives, trouble breathing, facial redness and rash.
The most common side effects of VYEPTI are stuffy nose and scratchy throat (nasopharyngitis) and allergic reactions.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 40 years of age. The number of patients older than 65 years was insufficient to determine if the occurrence of side effects in this age group differ from the younger patients.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved VYEPTI based primarily on evidence from two clinical trials (Trial 1/ NCT02559895 and Trial 2/ NCT02974153) of 1741 patients with chronic or episodic migraine headaches. Trials were conducted at 212 sites in United States, Georgia, Russia, Ukraine and European Union.
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Adapted from FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
*Other includes Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander Multiple and Other
Adapted from FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Adapted from FDA Review
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trials.
Figure 4. Baseline Demographics by Ethnicity
Adapted from FDA Review
How were the trials designed?
The benefit and side effects of VYEPTI were evaluated in two clinical trials of adult patients 18 – 71 years of age with a history of migraine headaches. The trials had similar designs.
Trial 1 enrolled patients with a history of episodic migraine headaches and Trial 2 enrolled patients with chronic migraine headaches. Patients were assigned to receive one of two doses of VYEPTI or placebo injections every 3 months for a total of 12 months in Trial 1, and for a total of 6 months in Trial 2. Neither the patients nor the health care providers knew which treatment was being given until the trial was completed.
The benefit of VYEPTI in comparison to placebo was assessed based on the change in the number of migraine days per month during the first 3-month treatment period.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
























.png)












No hay comentarios:
Publicar un comentario