
La FDA reconoce las consecuencias significativas para la salud pública que pueden resultar de la escasez de medicamentos y hace un gran esfuerzo dentro de sus facultades legales para abordar yprevenir la escasez de medicamentos. La escasez se produce por muchas razones, incluyendoproblemas de fabricación y calidad, retrasos y discontinuación del producto. Cuando los problemas son descubiertos por la empresa o el público y reportados a la FDA o se descubren por inspecciones de la FDA, la FDA trabaja en estrecha colaboración con la empresa para hacer frente a los riesgosinvolucrados y evitar daños a los pacientes. La FDA también considera el impacto que una escaseztendría en la atención médica del paciente y al acceso del producto y trabaja con la empresa pararestablecer el suministro al tiempo que garantiza la seguridad de los pacientes. Más información

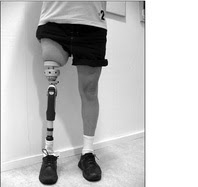
FDA authorizes use of prosthesis for rehabilitation of above-the-knee amputations
FDA has authorized use of the first prosthesis marketed in the U.S. for adults who have amputations above the knee and who have rehabilitation problems with, or cannot use, a conventional socket prosthesis. The Osseoanchored Prostheses for the Rehabilitation of Amputees (OPRA) device instead uses fixtures and screws implanted into the patient’s remaining thigh bone to connect an external prosthetic limb. More information
FDA has recently approved the Ventana ALK (D5F3) CDx Assay
The VENTANA ALK (D5F3) CDx Assay is a laboratory immunohistochemical (IHC) test that identifies whether the anaplastic lymphoma kinase (ALK) protein is present in a non-small cell lung cancer (NSCLC) tissue sample. If the test result indicates that the ALK protein is present, then the patient with NSCLC may be eligible for treatment with the cancer drug Xalkori® (crizotinib). Xalkori is a drug used to treat patients with advanced (locally or metastatic) NSCLC who have ALK positive tumors. Xalkori selectively interferes with the ALK protein and stops cancer cell growth in NSCLC.
More information
FDA approves targeted therapy for first-line treatment of patients with a type of metastatic lung cancer
FDA has approved Iressa (gefitinib) for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors harbor specific types of epidermal growth factor receptor (EGFR) gene mutations, as detected by an FDA-approved test. Lung cancer is the leading cause of cancer-related death among men and women in the U.S. and, though more common in men, the number of deaths from lung cancer in women is increasing. According to the National Cancer Institute, an estimated 221,200 Americans will be diagnosed with lung cancer, and 158,040 will die from the disease this year. NSCLC is the most common type of lung cancer. Mutations in the EGFR gene are present in about 10 percent of NSCLC tumors.
More information
FDA approves new drug to treat schizophrenia and as an add on to an antidepressant to treat major depressive disorder
FDA has approved Rexulti (brexpiprazole) tablets to treat adults with schizophrenia and as an add-on treatment to an antidepressant medication to treat adults with major depressive disorder (MDD). Schizophrenia is a chronic, severe, and disabling brain disorder affecting about one percent of Americans. Typically, symptoms are first seen in adults younger than 30 years of age and include hearing voices; believing other people are reading their minds or controlling their thoughts; and being suspicious or withdrawn.
More information
|
For information on drug approvals or to view prescribing information and patient information, please visit Drugs@FDA or DailyMed.




More Collaboration, Research Needed to Develop Cures, by Robert Califf, M.D., FDA's Deputy Commissioner for Medical Products and Tobacco
The U.S. Food and Drug Administration’s drug approval process—the final stage of drug development—is the fastest in the world, which means Americans typically have first access to new drugs when they are demonstrated to be safe and effective. But even as our agency has transformed the approval process—approving 51 new molecular entities and biological products last year alone, including more new orphan drugs for rare diseases than in any previous year—drug discovery and development is not keeping pace for many diseases. In many cases, what’s holding back progress is a lack of understanding of the biology of disease, as we outline in a new report we are releasing today that compares diseases where there is a robust pipeline of new therapies with certain diseases that have few known treatments or cures.
FDA advisory committee meetings are free and open to the public. No prior registration is required to attend. Interested persons may present data, information, or views, orally at the meeting, or in writing, on issues pending before the committee.
Other types of meetings listed may require prior registration and fees.
Public Meeting: Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee Meeting Announcement
Date: September 24, 2015
The committee will discuss the risks and benefits of Bayer HealthCare’s Essure System for permanent female sterilization. The system, originally approved in November 2002, under P020014, consists of a delivery system and nickel-containing permanent implants. The implants are placed without a skin incision, through the vagina, within each fallopian tube; they elicit tissue ingrowth, which over time results in tubal occlusion. More information
|

Please visit FDA’s Advisory Committee page to obtain advisory committee meeting agendas, briefing materials, and meeting rosters prior to the meetings. You may also visit this page after meetings to obtain transcripts, presentations, and voting results.
For additional information on other agency meetings please
visit Meetings, Conferences, & Workshops.


FDA Strengthens Warning of Heart Attack and Stroke Risk for Non-Steroidal Anti-Inflammatory Drugs
Next time you reach into the medicine cabinet seeking relief for a headache, backache or arthritis, be aware of important safety information for non-steroidal anti-inflammatory drugs. FDA is strengthening an existing warning in prescription drug labels and over-the-counter (OTC) Drug Facts labels to indicate that nonsteroidal anti-inflammatory drugs (NSAIDs) can increase the chance of a heart attack or stroke, either of which can lead to death. Those serious side effects can occur as early as the first few weeks of using an NSAID, and the risk might rise the longer people take NSAIDs. (Although aspirin is also an NSAID, this revised warning doesn’t apply to aspirin.) The OTC drugs in this group are used for the temporary relief of pain and fever. The prescription drugs in this group are used to treat several kinds of arthritis and other painful conditions. Because many prescription and OTC medicines contain NSAIDs, consumers should avoid taking multiple remedies with the same active ingredient.
More information
|
More Consumer Updates
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products.
More information
En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización.
En Español
|
|


































.png)












No hay comentarios:
Publicar un comentario