Volume 26, Number 12—December 2020
Dispatch
Antibody Profiles According to Mild or Severe SARS-CoV-2 Infection, Atlanta, Georgia, USA, 2020
On This Page
Downloads
Article Metrics
William T. Hu , J. Christina Howell, Tugba Ozturk, Karima Benameur, Leda C. Bassit, Richard Ramonell, Kevin S. Cashman, Shama Pirmohammed, John D. Roback, Vincent C. Marconi, Irene Yang, Valerie V. Mac, Daniel Smith, Ignasio Sanz, Whitney Wharton, F. Eun-Hyung Lee, and Raymond F. Schinazi
, J. Christina Howell, Tugba Ozturk, Karima Benameur, Leda C. Bassit, Richard Ramonell, Kevin S. Cashman, Shama Pirmohammed, John D. Roback, Vincent C. Marconi, Irene Yang, Valerie V. Mac, Daniel Smith, Ignasio Sanz, Whitney Wharton, F. Eun-Hyung Lee, and Raymond F. Schinazi
Abstract
Among patients with coronavirus disease (COVID-19), IgM levels increased early after symptom onset for those with mild and severe disease, but IgG levels increased early only in those with severe disease. A similar pattern was observed in a separate serosurveillance cohort. Mild COVID-19 should be investigated separately from severe COVID-19.
Coronavirus disease (COVID-19) emerged in December 2019 (1,2), and by June 2020, »10 million persons worldwide had acquired the disease. The confirmatory test for severe acute respiratory syndrome virus 2 (SARS-CoV-2) infection remains real-time reverse transcription PCR, but this test poses challenges in terms of sensitivity (3), reagent or equipment availability, and specialized personnel training. Serologic assays can be readily performed in most clinical laboratories, with faster turnaround times, but their association with COVID-19 has largely been reported for hospitalized patients with severe disease (4; E. Adams et al., unpub. data, ). Whether mild and severe COVID-19 represent 2 interlinked stages on a severity continuum or 2 distinct phenotypes of an infectious process (5) remains incompletely understood; detailed cross-sectional characterization of IgM and IgG reactive against SARS-CoV-2 antigens may provide insight into the temporal evolution of antibodies. Detection of cross-reactive antibodies from a pre-2020 cohort can also indicate whether past exposure to other coronaviruses is associated with cross-reactive protection against SARS-CoV-2.
In addition to IgG targeting the receptor-binding domain (RBD) of the spike protein subunit S1 (6), we developed and validated an IgM assay targeting the full-length S1 protein. We further developed and validated an IgM assay targeting the small full-length envelope (E) protein, which is highly shared between SARS-CoV and SARS-CoV-2 (2), is accessible on the surface, and increases during virus replication (7). Using these assays, we characterized the IgM and IgG profiles of participants with COVID-19, pre-2020 control participants, and a community cohort of 116 persons who had recovered from self-limited illness during March and April 2020 in Atlanta, Georgia, USA.
We recruited 28 participants hospitalized for severe COVID-19 (20 requiring artificial ventilation; samples collected during hospitalization a median of 15.5 days after symptom onset) and 15 participants who had recently recovered from mild COVID-19 (samples collected a median of 15 days after symptom onset; Table 1). Compared with hospitalized participants, participants with mild illness were less likely to be African American (8) and more likely to be younger and to have nasal congestion or anosmia.
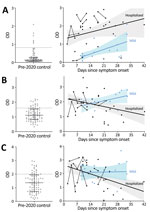
Compared with control participants, hospitalized participants had higher levels of IgG against S1-RBD (log10 transformed because of nonnormal distribution; Student t [56.7] = 12.183; p<0.0001; Figure 1, panel A), IgM against S1 (Student t [33.29] = 3.713; p<0.001; Figure 1, panel B), and IgM against E (t [129] = 2.279; p = 0.024; Figure 1, panel C). The same was true among participants with mild illness for IgG against S1-RBD (Student t [116] = 4.246; p<0.0001; Figure 1, panel A), IgM against S1 (Student t [116] = 6.764; p<0.0001; Figure 1, panel B), and IgM against E (Student t [116] = 3.398; p = 0.001; Figure 1, panel C). However, an IgG diagnostic threshold of 0.82 optical density (OD) (Appendix) from the hospitalized participants identified only 4 (26.7%) of 15 participants with mild disease because of the lower IgG levels early after symptom onset in the group with mild disease. Elevated IgG only weeks after symptom onset among participants with mild COVID-19 is consistent with prior reports (9; E. Adams et al., unpub. data, ), and linear regression analysis projected that their IgG would reach the threshold of hospitalized participants an average of 29 days after symptom onset.
Conversely, IgM negatively correlated with time since symptom onset for hospitalized participants but not for those with mild disease. An anti-S1 IgM level of 1.60 OD from hospitalized patients during the first 21 days—before significant IgM decline—and 50-fold randomly selected control participants showed sensitivity of 81.0% and median specificity of 80.4% (range 76%–85.5%). The threshold of 1.60 OD was in range with values derived from pre-adsorption experiments that used S1 antigen (1.75 OD; Appendix) and identified participants with mild disease with sensitivity of 80.0% and median specificity of 80.5% (range 80%–86.7%). Anti-E IgM levels showed similar associations with time from symptom onset and severity but did not increase identification of COVID-19 participants.
Because many persons with mild influenza-like (ILI) symptoms in the metropolitan Atlanta area did not or could not access SARS-CoV-2 testing during early 2020, we also analyzed antibody levels in 116 adults who had recovered from self-limited ILI symptoms (Table 2). Compared with participants with mild COVID-19, this cohort was less likely to have anosmia (11% vs. 47%; p = 0.002) or fatigue (4% vs. 20%; p = 0.048) but was otherwise similar in terms of sex, race, age, and signs/symptoms. Of 31 participants with symptom onset 7–29 days before blood collection, 1 (3%) had elevated IgG, and 11 (12.9%) of 85 with symptom onset 30–60 days before participation had elevated IgG. None of the clinical signs/symptoms strongly predicted antibody levels. A liberal threshold of anti-S1 IgM >1.60 OD identified 18/31 (58%) and 57/85 (67%) participants, and a more stringent threshold of 2.00 OD to reduce false positives identified 7/31(22%) and 41/85 (48%) participants.
Last, we performed plaque-reduction neutralization assays (PRNT; Appendix) for a subgroup of participants with confirmed or probable COVID-19 and pre-2020 control participants (75% with elevated antibody levels; Figure 2, panel A). All 6 hospitalized participants and 5 participants with mild disease (2 weak neutralizing results <1:40) demonstrated >90% plaque reduction in Vero cells compared with 2 of 15 control participants who also showed weak neutralization. Using positive PRNT at >1:40 as a specific threshold, we found simultaneously elevated IgM and IgG most predictive of positive PRNT (p = 0.008 compared with IgM alone, p = 0.07 compared with IgG alone; Appendix), although plasma from 1 hospitalized participant with neutralizing plasma had reference IgM and IgG levels. PRNT for community participants with the 10 most elevated IgG levels showed a similar trend (Figure 2, panel B).
IgM reactive toward S1 and E proteins increased early regardless of disease severity, but IgG increased early only in hospitalized participants with severe COVID-19. This pattern was observed in a separate cohort of community participants who had recovered from self-limited ILI. Positive PRNT—a surrogate for antibody-mediated immune protection—may be better associated with elevated IgM and IgG than either antibody alone.
A diagnostic algorithm of IgG from hospitalized participants performed poorly for detection of mild COVID-19. Similarly, other studies found delayed or low-to-medium neutralizing antibody titers in persons who recovered from mild COVID-19 (E. Adams et al., unpub. data, ; F. Wu et al., unpub. data, ). The delayed increase in IgG and neutralizing antibodies in persons with mild COVID-19 also suggests that mild cases do not necessarily represent an intermediate stage between severe and asymptomatic COVID-19. A corollary of slow IgG increases in persons with mild COVID-19 may be longer persistence of IgM, but more definitive characterization of IgM+ memory B cells (10) and long-term decay of antibody levels (11) is needed.
Our study has limitations. Our small cross-sectional cohort of patients with well-characterized and laboratory-confirmed COVID-19 limits generalization. The overrepresentation of African Americans in the more severely ill cohort may mediate some differences in antibody profiles (8), and we did not measure IgA levels or antibodies targeting other SARS-CoV-2 gene products (currently under development and validation). We also did not measure antibody levels in historic SARS or MERS case-patients, and cross-reactive antibody response against homologous regions cannot be ruled out.
We did confirm a complex relationship between antibody levels, disease severity, and time since symptom onset. Examining IgM and IgG against multiple SARS-CoV-2–related antigens may thus better inform natural history and vaccine studies than any one antibody.
Dr. Hu is a physician-scientist at Emory University in Atlanta, GA. His research interests involve reliable fluid biomarkers for human diseases related to inflammation.
Acknowledgments
This article was preprinted at https://www.medrxiv.org/content/10.1101/2020.05.10.20097535v1
This work was supported by National Institutes of Health grants R01 AG 054046, R01 AG054991, and T32HL116271.
W.T.H. and Emory University have licensed the IgM assay panel for SARS-CoV-2, have a patent on the cerebrospinal fluid–based diagnosis of frontotemporal lobar degeneration with TDP-43 inclusions, and have a patent pending on the cerebrospinal fluid–based prognosis of spinal muscular atrophy. W.T.H. has consulted for ViveBio, LLC; AARP, Inc.; and Biogen, Inc. and has received research support from Fujirebio US. F.E.-H.L. is the founder of MicroB-plex, Inc., and has research grants with Genentech, Inc.
References
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–9.
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020.
- Amanat F, Nguyen THO, Chromikova V, Strohmeier S, Stadlbauer D, Javier A, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. 2020;26:1033–6.
- Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–88.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–20.
- Nieto-Torres JL, Dediego ML, Alvarez E, Jiménez-Guardeño JM, Regla-Nava JA, Llorente M, et al. Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology. 2011;415:69–82.
- Bernard NJ. Double-negative B cells. Nat Rev Rheumatol. 2018;14:684.
- Bao M, Zhang Y, Wan M, Dai L, Hu X, Wu X, et al. Anti-SARS-CoV immunity induced by a novel CpG oligodeoxynucleotide. Clin Immunol. 2006;118:180–7.
- Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–7.
- Liu X, Wang J, Xu X, Liao G, Chen Y, Hu CH. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg Microbes Infect. 2020;9:1269–74.
Figures
Tables
Suggested citation for this article: Hu WT, Howell JC, Ozturk T, Benameur K, Bassit LC, Ramonell R, et al. Antibody profiles according to mild or severe SARS-CoV-2 infection, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 2020 Dec [date cited]. https://doi.org/10.3201/eid2612.203334
Original Publication Date: August 28, 2020






















.png)












No hay comentarios:
Publicar un comentario