
Volume 26, Number 5—May 2020
Research Letter
Crimean-Congo Hemorrhagic Fever Virus Antibodies among Livestock on Corsica, France, 2014–2016
On This Page
Figures
Altmetric
Sébastien Grech-Angelini , Renaud Lancelot, Olivier Ferraris, Christophe Nicolas Peyrefitte, Nathalie Vachiery, Aurélie Pédarrieu, Armelle Peyraud, Valérie Rodrigues, Denise Bastron, Geneviève Libeau, Bernard Fernandez, Philippe Holzmuller, Renata Servan de Almeida, Vincent Michaud, Noël Tordo, Loïc Comtet, Raphaëlle Métras, François Casabianca, and Laurence Vial
, Renaud Lancelot, Olivier Ferraris, Christophe Nicolas Peyrefitte, Nathalie Vachiery, Aurélie Pédarrieu, Armelle Peyraud, Valérie Rodrigues, Denise Bastron, Geneviève Libeau, Bernard Fernandez, Philippe Holzmuller, Renata Servan de Almeida, Vincent Michaud, Noël Tordo, Loïc Comtet, Raphaëlle Métras, François Casabianca, and Laurence Vial
Abstract
We conducted a serologic survey for Crimean-Congo hemorrhagic fever virus antibodies in livestock (cattle, sheep, and goats; N = 3,890) on Corsica (island of France) during 2014–2016. Overall, 9.1% of animals were seropositive, suggesting this virus circulates on Corsica. However, virus identification is needed to confirm these results.
Crimean-Congo hemorrhagic fever (CCHF), the most widespread tickborne viral infection in humans, is a zoonotic disease caused by an orthonairovirus of the Nairoviridae family. Symptoms in humans vary from a nonspecific mild febrile syndrome to severe hemorrhagic disease that sometimes leads to death (1,2), and a wide range of animals are asymptomatic reservoirs (1). Corsica is an island of France located in the northwestern part of the Mediterranean Sea (Figure, panel A). Entomologic surveys have revealed that one of the main vectors of CCHF virus (CCHFV), the Hyalomma marginatum tick, is abundant on this island (1,3,4). Therefore, we performed a serologic cross-sectional survey to assess the prevalence of antibodies against CCHFV in domestic ruminants on Corsica. This work was approved by the French Ministry of Agriculture (Direction Départementale de la Cohésion Sociale et de la Protection des Populations of Corse-du-Sud and Haute-Corse and General Directorate for Food).
As part of national surveillance for animal diseases, veterinarians collected cattle, goat, and sheep blood samples during 2014–2016. In total, 3,890 animals (1,731 cattle, 1,035 goats, 1,124 sheep) were sampled from 269 farms, originating from 46% (137/298) of the municipalities with ruminant farming activities (3).
We tested the collected serum samples for the presence of CCHFV IgG using a double-antigen ELISA kit (ID Screen CCHF Double Antigen Multi-species, ID.Vet, ) according to the manufacturer’s instructions (Appendix Figure). For this kit, the 95% CI for sensitivity is 96.8%–99.8%, and 95% CI for specificity is 99.8%–100% (5). To confirm ELISA results, we sent 35 ELISA-positive and 5 ELISA-negative serum samples to a Biosafety Level 4 laboratory (Laboratory Jean Mérieux, Lyon, France) to be analyzed by the World Health Organization and World Organisation for Animal Health national reference center for CCHFV (Institut Pasteur and Institut de Recherche Biomédicale des Armées, Paris, France). We used the pseudo–plaque reduction neutralization test (PPRNT) (6) to measure the neutralizing antibodies against IbAr10200 (same antigen used in ELISA) in triplicate. We included Hazara virus (same serogroup as CCHFV) and Dugbe virus (closely related virus, Nairobi sheep disease serogroup) to detect possible immune cross-reactions. We estimated overall and species-specific IgG prevalence against CCHFV using a β-binomial logistic regression model of data grouped by farm.
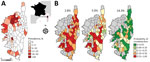
The overall estimated seroprevalence was 9.1% (95% CI 6.9%–11.9%); estimated seroprevalence in cattle was 13.3% (95% CI 10.2%–17.3%), goats 3.1% (95% CI 1.4%–7.0%), and sheep 2.5% (95% CI 1.0%–5.9%). CCHFV antibodies were detected across the island; 35.8% (49/137; 95% CI 27.8%–44.4%, estimated by exact binomial test) of the investigated municipalities had >1 positive ELISA test result. Because serum samples were not available from all municipalities, we used Voronoi polygons to draw regional boundaries and estimate the spatial distribution of seroprevalence across the island. Seroprevalence was high in the northwest corner of Corsica; however, most regions lacked evidence of seropositivity (Figure panel A). In areas corresponding to negative polygons, the probability of nondetection of positive serum samples was estimated assuming 3 levels of estimated seroprevalence corresponding with the 10% quantile (2.8% seroprevalence), 25% quantile (5.0% seroprevalence), and 50% quantile (14.3% seroprevalence) (Figure, panel B) and by accounting for sample size. This data shows that if seroprevalence in these regions is <5%, the probability of nondetection is high (Figure, panel B), and if the seroprevalence in these regions is >14.3%, the probability of nondetection is low. Therefore, the chance that we missed hotspots of transmission is highly unlikely.
Of 35 ELISA-positive serum samples tested, none showed neutralizing antibodies against Hazara and Dugbe viruses, and no ELISA-negative serum sample showed neutralizing antibodies against CCHFV, Hazara virus, or Dugbe virus (at lowest dilution 1:20; Appendix Table). Of 35 ELISA-positive serum samples, 23 had neutralizing antibodies against CCHFV at the 1:40 dilution, and 10 remained positive at the 1:80 dilution (including 2 positive at the 1:320 and 1:640 dilutions).
Our serologic survey results suggest CCHFV circulates in livestock on Corsica. Relative discrepancies between ELISA (35 positives) and PPRNT (23 positives) findings might result from their different target epitopes; the ELISA measures total immunoglobulin (neutralizing and nonneutralizing antibodies) and PPRNT just a subset (functional neutralizing antibodies) (7). Seroprevalence estimates were higher in cattle than smaller ruminants, probably reflecting that cattle in Corsica are more infested by Hy. marginatum ticks (3).
As of February 2020, CCHFV has not been detected in ticks on Corsica (8), and no clinical human case has been reported. The presence of a genetically close and less virulent strain in ticks on Corsica might help explain the lack of these findings. CCHFV was detected in ticks in Spain, where the first human cases were reported in 2016 (9), and in a tick collected on a migratory bird in Italy (10). Entomologic and epidemiologic investigations to identify the incriminated strain and characterize its spatial distribution are ongoing. This work will be essential to assess the risk for human CCHFV exposure and raise public health awareness on Corsica and in neighboring areas.
Dr. Grech-Angelini is a veterinary epidemiologist for the Groupement Technique Vétérinaire de Corse, Ghisonaccia, France, who obtained his doctorate during the completion of this study in 2020 with the Animal Health Laboratory of Unité Mixte de Recherche de Biologie Moléculaire et d’Immunologie Parasitaires (Agence Nationale de Sécurité Sanitaire de l’Alimentation de l’Environnement et du Travail, Institut National de la Recherche Agronomique, École Nationale Vétérinaire d’Alfort, Maisons-Alfort, France). His primary research interests are the epidemiology of zoonoses and tickborne diseases.
Acknowledgments
The authors thank staff of the 2 district veterinary laboratories of Corsica for their help in collecting blood samples.
This study was partially funded by the French Ministry of Agriculture General Directorate for Food.
References
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–89.
- Dreshaj S, Ahmeti S, Ramadani N, Dreshaj G, Humolli I, Dedushaj I. Current situation of Crimean-Congo hemorrhagic fever in Southeastern Europe and neighboring countries: a public health risk for the European Union? Travel Med Infect Dis. 2016;14:81–91.
- Grech-Angelini S, Stachurski F, Lancelot R, Boissier J, Allienne JF, Marco S, et al. Ticks (Acari: Ixodidae) infesting cattle and some other domestic and wild hosts on the French Mediterranean island of Corsica. Parasit Vectors. 2016;9:582.
- Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antiviral Res. 2017;144:93–119.
- Sas MA, Comtet L, Donnet F, Mertens M, Vatansever Z, Tordo N, et al. A novel double-antigen sandwich ELISA for the species-independent detection of Crimean-Congo hemorrhagic fever virus-specific antibodies. Antiviral Res. 2018;151:24–6.
- Canakoglu N, Berber E, Ertek M, Yoruk MD, Tonbak S, Bolat Y, et al. Pseudo-plaque reduction neutralization test (PPRNT) for the measurement of neutralizing antibodies to Crimean-Congo hemorrhagic fever virus. Virol J. 2013;10:6.
- Ravault S, Friel D, Di Paolo E, Caplanusi A, Gillard P, Povey M, et al. Assessment of mumps virus-specific antibodies: comparison of plaque reduction neutralization test and enzyme-linked immunosorbent assay estimates. J Infect Dis. 2019;220:1462–8.
- Grech-Angelini S, Stachurski F, Vayssier-Taussat M, Devillers E, Casabianca F, Lancelot R, et al. Tick-borne pathogens in ticks (Acari: Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transbound Emerg Dis. 2019;
tbed.13393 . - Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, et al.; Crimean Congo Hemorrhagic Fever@Madrid Working Group. Autochthonous Crimean-Congo hemorrhagic fever in Spain. N Engl J Med. 2017;377:154–61.
- Mancuso E, Toma L, Polci A, d’Alessio SG, Di Luca M, Orsini M, et al. Crimean-Congo hemorrhagic fever virus genome in tick from migratory bird, Italy. Emerg Infect Dis. 2019;25:1418–20.
Figure
Cite This ArticleOriginal Publication Date: 4/9/2020





















.png)












No hay comentarios:
Publicar un comentario